Serum lipoprotein(a) [Lp(a)] is a coronary artery disease (CAD) risk factor in persons of European ancestry. Levels are twofold to threefold higher in African-Americans (AAs), but reported associations with CAD have been inconsistent. The relation of Lp(a) with the extent and severity of subclinical coronary plaque has not been described in AAs. We screened 269 apparently healthy AAs for risk factors and coronary plaque using advanced coronary computed tomographic angiography. Total coronary plaque (TCP), noncalcified coronary plaque, and calcified coronary plaque volumes (mm 3 ) were quantified using a validated automated method. Lp(a) was measured by ELISA. Multivariable modeling was performed with adjustment for traditional CAD risk factors and intrafamilial correlations. Mean age was 51 ± 11 years and 64% were female. Plaque was present in 41%. Lp(a) was independently associated with TCP volume [log(TCP + 1)] (p = 0.04), 3-vessel and/or left main involvement (p = 0.04), and at least 1 stenosis >50% (p = 0.006). Best-fit regression analyses showed that subjects with Lp(a) >40 mg/dl were threefold more likely to have 3-vessel and/or left main disease (95% confidence interval 1.4 to 6.8, p = 0.005) and fourfold more likely to have stenosis >50% (95% confidence interval 1.3 to 15.0, p = 0.02). In subjects with plaque (n = 110), multivariable models showed the Lp(a) level was significantly and independently associated with TCP (p = 0.009), noncalcified coronary plaque (p = 0.01), and calcified coronary plaque (p = 0.003) and affected vessel length (p = 0.01). In conclusion, high Lp(a) is strongly associated with coronary plaque volumes, extent, and severity in apparently healthy AAs. High levels of Lp(a) may be particularly important in the pathogenesis of CAD in AAs.
To more accurately assess the relation of Lp(a) with preclinical coronary atherosclerosis in younger African Americans (AAs), who have significantly more noncalcified coronary plaque (NCP) than calcified coronary plaque (CCP), we designed this study to examine the association of Lp(a) with the volume, extent, and severity of coronary plaque using advanced multidetector computed tomographic angiography (CTA) in healthy asymptomatic AAs from early-onset coronary artery disease (CAD) families.
Methods
The population consisted of 269 participants in the Early Noninvasive Imaging for Genetic Mechanisms of Atherosclerosis substudy of the larger ongoing Genetic Study of Atherosclerosis Risk (GeneSTAR), a prospective study designed to characterize genetic and biologic factors associated with cardiovascular disease phenotypes in families with early-onset CAD. Probands <60 years of age with documented acute myocardial infarction, unstable angina with coronary revascularization, or acute angina with angiographic evidence of a flow-limiting stenosis of >50% diameter in at least 1 coronary artery were identified during hospitalization and excluded. Apparently healthy siblings and the offspring of the probands and siblings were eligible if they were 30 to 75 years of age and had no known personal history of CAD. Siblings and offspring were excluded if they had systemic autoimmune disease, known allergy to iodinated contrast media, or chronic kidney disease. The study was approved by the Johns Hopkins Medicine Institutional Review Board, and all participants gave informed consent.
Participants underwent a comprehensive risk factor screening after a 12-hour overnight fast. Medical history and current medication use were elicited. A physical examination was performed by a study physician. Height was determined using a fixed stadiometer, and weight was measured on a balance beam scale with the subject wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg)/height (m) 2 . Current cigarette smoking behavior was assessed by self-report and verified by expired carbon monoxide levels of ≥8 ppm. Blood pressure was measured according to the American Heart Association guidelines 3 times over the course of the day. Hypertension was defined as an average blood pressure ≥140 mm Hg systolic, ≥90 mm Hg diastolic, and/or use of an antihypertensive drug. Blood was obtained, and total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using the US Centers for Disease Control standardized methods. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula for subjects with triglyceride levels up to 400 mg/dl. Direct measurement of LDL cholesterol using ultracentrifugation was used for subjects with triglyceride levels ≥400 mg/dl (n = 2). Glucose concentration was measured using the glucose oxidase method; type 2 diabetes was defined as a physician-diagnosed history, a fasting glucose level ≥126 mg/dl, and/or use of prescribed hypoglycemic medications. After blood collection, plasma samples were sent to the University of Maryland Cytokine Core for analysis. Lp(a) was analyzed using a Human Lp(a) ELISA kit (ALerCHEK, Inc., Springvale, Maine). This assay uses a solid-phase capture sandwich ELISA that measures human Lp(a) independent of isoform size. The intra-assay coefficient of variation has been shown to be 4% to 8%.
All participants underwent coronary CTA using a newest generation dual-source multidetector scanner (SOMATOM Definition Flash; Siemens Medical Solutions, Forchheim, Germany). Because of the high temporal resolution and excellent image quality of the scanner, β blockade was not necessary for reducing the heart rate. A noncontrast scan was first performed to determine the coronary artery calcium volume. Subsequently, coronary CTA was performed to examine the presence, location, composition, and severity of any coronary plaque. Approximately 80 ml of iso-osmolar contrast agent (320 mg iodine/ml) was injected at 6 ml/s. Prospective electrocardiographic gating was used in patients with low, steady heart rates (<65 beats/min) and little heart rate variability. For patients with variable heart rates or heart rates >65 beats/min, retrospective gating with dose modulation was used. Tube potential was selected on a per-patient basis by the performing technologist’s assessment of patient size; 100 kV was used for patients who were not overweight or obese, otherwise 120 kV was used. We reconstructed 0.75-mm-thick axial slices at 0.4-mm intervals with a B26 kernel; 10 reconstructions were done at 10% increments in the RR interval. All scans were evaluated with the CT radiologist blinded to the participants’ risk factor profiles. The coronary arterial tree was segmented according to the standard American Heart Association classification, and the segments were investigated for plaque and luminal narrowing. Any focal stenosis >50% in severity was identified using quantitative software (COR Analyzer System; Rcadia Medical Imaging, Haifa, Israel) and verified by the expert reader.
The volume of CCP was measured on a workstation (Leonardo Multimodality Workstation, Syngo; Siemens Medical Solutions, Malvern, Pennsylvania) using noncontrast images. Regions of interest were placed over each of the coronary arteries, and a threshold of >130 HU was used for determining per-vessel volumes of CCP (mm 3 ) using standard validated methods. Vessel CCP volumes were summed for a total CCP volume. For each affected coronary segment, NCP volumes (mm 3 ) were quantified using AutoPlaq (Cedars-Sinai Medical Center, Los Angeles, California), as previously described. This automated method of NCP measurement has high interobserver correlation (r = 0.97) and has been previously validated against intravascular ultrasound. To quantify each affected segment, CTA images were examined in multiplanar format and proximal and distal limits of the plaque were manually marked. Control points defining the lumen center line were placed. Subsequent NCP plaque quantification was then fully automated using adaptive algorithms that are scan specific per individual. Segmental NCP volumes were summed for a total NCP volume per vessel, including the left main (LM), left anterior descending, left circumflex, and right coronary arteries. The vessel-specific volumes were summed for a total NCP volume. Total coronary plaque (TCP) was calculated as the sum of CCP + NCP. In addition, affected coronary segment length with any plaque was automatically quantified and summed for total vessel length affected (mm).
Standard descriptive analyses were used to examine distributions of sociodemographic and CAD risk factor variables by race and the absence or presence of coronary plaque. The Kolmogorov-Smirnov statistic was used to test for normality of continuous variables. Given the skewed distribution of Lp(a), log(Lp(a)) was used to compare subjects with and without plaque. In subjects with any coronary plaque, the correlation of Lp(a) with TCP, CCP, and NCP volumes was examined. Mixed multivariable regression models were performed predicting transformed plaque volumes as log(plaque volume) and affected vessel length, accounting for nonindependence within families as a random effect. Covariates in the model included age, gender, the presence of hypertension and/or diabetes, current smoking behavior, HDL cholesterol, LDL cholesterol, triglycerides, BMI, education level, and Lp(a). To examine the association of Lp(a) with plaque presence and volume in the entire population, TCP volumes were transformed as log [TCP + 1], given the nonnormal distribution and presence of zero plaque in many subjects. Mixed multivariable regression analysis was performed using the same covariates. Logistic regressions predicting the presence of 3-vessel and/or LM disease and the presence of at least 1 stenosis >50% were performed separately with the same dependent variables using Lp(a) as a continuous variable. Maximum likelihood analyses were performed for each model to determine the best-fit Lp(a) risk level, for which odds ratios were determined.
Results
Apparently healthy subjects (n = 269) from 147 families with the onset of CAD <60 years of age agreed to participate. Study subjects were siblings (n = 166) of the index patient or adult offspring of the index patient or the siblings (n = 103). The sample was 64.3% female with a mean age of 51.3 ± 11.1 years. All were healthy and without any chest pain or angina-equivalent symptoms. Overall, 40.9% of the subjects had subclinical coronary plaque, including 5.6% with exclusively NCP without any CCP. Population demographics and CAD risk factors and the absence or presence of coronary plaque are listed in Table 1 . The presence of coronary plaque was significantly associated with older age, hypertension, diabetes, and triglyceride level. Median Lp(a) level was higher in subjects with plaque than in those with no plaque, but this difference was not statistically significant. There were no significant gender differences in the Lp(a) level in subjects with or without plaque.
| Variable | No Plaque (N=159) | Plaque (N=110) | p-value |
|---|---|---|---|
| Age (years) | 47.1 ± 10.8 | 57.4 ± 8.2 | <0.0001 |
| Men | 28.9% | 45.4% | 0.005 |
| Hypertension | 43.4% | 71.8% | <0.0001 |
| Diabetes mellitus | 12.6% | 25.4% | 0.007 |
| Current smoker | 23.9% | 28.2% | 0.43 |
| Statin use | 14.5% | 38.2% | <0.0001 |
| LDL cholesterol (mg/dl) | 109.9 ± 36.8 | 117.3 ± 38.9 | 0.11 |
| HDL cholesterol (mg/dl) | 58.5 ± 15.4 | 58.5 ± 17.2 | 1.00 |
| Triglycerides (mg/dl) | 91.1 ± 48.7 | 111.2 ± 80.5 | 0.01 |
| Body-mass index (kg/m 2 ) | 31.8 ± 7.2 | 31.6 ± 6.0 | 0.87 |
| Education (years) | 13.2 ± 2.4 | 13.0 ± 2.7 | 0.77 |
| Lp(a) (mg/dl) † | 19.5 [14.2,40.0] | 25.8 [13.9,49.7] | 0.13 |
∗ Continuous variables presented as mean ± 1 SD.
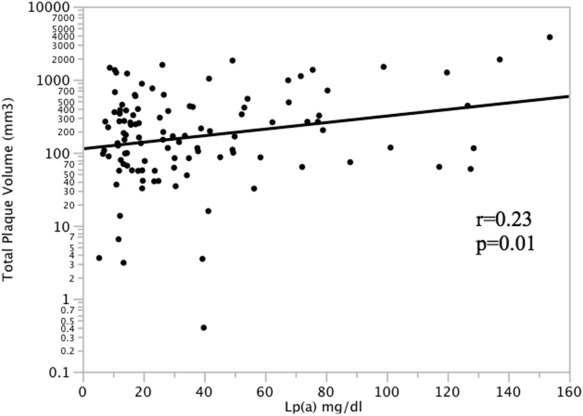
In participants with coronary plaque (n = 110), serum Lp(a) was significantly correlated with TCP volume ( Figure 1 ). Lp(a) was also significantly correlated with NCP volume (r = 0.20, p = 0.04), CCP volume (r = 0.31, p = 0.002), and total affected vessel length (r = 0.25, p = 0.01). Multivariate regression models accounting for intrafamilial correlation were performed in subjects with coronary plaque predicting each plaque phenotype using the covariates age, gender, hypertension, diabetes, current smoking, HDL cholesterol, LDL cholesterol, triglycerides, BMI, education, and Lp(a) level ( Table 2 ). Age and Lp(a) were significant predictors of total plaque volume. Age, male gender, and Lp(a) were significant predictors of NCP volume. Age, hypertension, and Lp(a) were significant predictors of CCP volume. Finally, age and Lp(a) were independently associated with total affected vessel length. Using the entire population (n = 269), multivariate regression models predicting the transformed TCP volume [log(TCP + 1)] were performed using the same covariates. Lp(a) level was significantly and independently associated with TCP (p = 0.04), as was older age (p <0.0001) and male gender (p = 0.0003).
| log(total plaque volume) | log(noncalcified volume) | log(calcified volume) | Affected length (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Beta ± SE | p-value | Beta ± SE | p-value | Beta ± SE | p-value | Beta ± SE | p-value | |
| Age | 0.056 ± 0.017 | 0.002 | 0.031 ± 0.014 | 0.02 | 0.090 ± 0.023 | 0.0002 | 1.553 ± 0.593 | 0.01 |
| Male sex | 0.154 ± 0.156 | 0.33 | 0.333 ± 0.123 | 0.008 | 0.011 ± 0.197 | 0.96 | 9.730 ± 5.411 | 0.08 |
| Hypertension | 0.099 ± 0.155 | 0.52 | 0.013 ± 0.119 | 0.91 | 0.527 ± 0.206 | 0.01 | 1.347 ± 5.266 | 0.80 |
| Diabetes mellitus | 0.125 ± 0.178 | 0.49 | 0.060 ± 0.135 | 0.65 | 0.037 ± 0.231 | 0.87 | 5.067 ± 5.994 | 0.40 |
| Current smoker | -0.017 ±0.169 | 0.92 | -0.115 ± 0.133 | 0.39 | -0.077 ± 0.221 | 0.73 | 2.021 ± 5.855 | 0.73 |
| LDL-C (mg/dl) | -0.002 ± 0.004 | 0.53 | -0.002 ± 0.003 | 0.44 | 0.003 ± 0.005 | 0.57 | -0.066 ± 0.128 | 0.61 |
| HDL-C (mg/dl) | 0.003 ± 0.009 | 0.77 | 0.010 ± 0.007 | 0.20 | 0.017 ± 0.012 | 0.16 | 0.436 ± 0.315 | 0.17 |
| Triglycerides (mg/dl) | 0.003 ± 0.002 | 0.17 | 0.003 ± 0.002 | 0.10 | 0.005 ± 0.003 | 0.08 | 0.123 ± 0.078 | 0.12 |
| Body-mass index (kg/m 2 ) | -0.034 ± 0.027 | 0.21 | -0.011 ± 0.020 | 0.57 | -0.104 ± 0.035 | 0.004 | -1.089 ± 0.894 | 0.23 |
| Education (years) | -0.050 ± 0.054 | 0.35 | -0.033 ± 0.042 | 0.44 | -0.048 ± 0.067 | 0.48 | -2.216 ± 0.139 | 0.24 |
| Lp(a) (mg/dl) | 0.011 ± 0.004 | 0.009 | 0.008 ± 0.003 | 0.02 | 0.018 ± 0.006 | 0.003 | 0.359 ± 0.139 | 0.01 |
Of those participants with coronary plaque, 43.6% had 3-vessel and/or LM artery involvement and 17.3% had at least 1 stenosis ≥50%. Median Lp(a) levels were significantly higher in AAs with extensive plaque (3-vessel and/or LM disease) than all others (32.5 mg/dl [13.9 to 70.8 mg/dl] vs 19.9 mg/dl [14.2 to 39.4 mg/dl], Wilcoxon p = 0.04). Similar results were found for severe plaque (at least 1 stenosis ≥50%) compared with all others (49.3 mg/dl [17.4 to 77.8 mg/dl] vs 20.6 mg/dl [14.1 to 40.4 mg/dl], Wilcoxon p = 0.01). In the fully adjusted model, continuous Lp(a) level was significantly associated with the number of coronary vessels affected (p = 0.05), the presence of 3-vessel and/or LM disease (p = 0.04), and at least 1 stenosis >50% in severity (p = 0.006). The maximum likelihood best fit for both models was Lp(a) >40 mg/dl ( Tables 3 and 4 ). Subjects with Lp(a) >40 mg/dl were approximately threefold more likely to have 3-vessel and/or LM plaque and fourfold more likely to have at least 1 stenosis >50% in severity.



