Hyperhomocysteinemia induces oxidative stress and endothelial dysfunction, which share the proposed pathophysiologic mechanisms of contrast-induced nephropathy (CIN). However, no study has investigated the relation between hyperhomocysteinemia and CIN. The aim of the present study was to evaluate the effects of hyperhomocysteinemia on CIN in patients undergoing percutaneous coronary intervention. This was an observational cohort study that included 572 patients who underwent percutaneous coronary intervention. CIN was defined as an absolute ≥0.5 mg/dl or a relative ≥25% increase in the serum creatinine level at 48 hours after the procedure. The incidence of CIN was significantly greater in patients in the third homocysteine tertile (from lowest to highest, 4.7%, 7.3%, and 24.2%, p <0.001). Furthermore, the homocysteine levels were significantly greater in patients with CIN than in those without CIN (16.9 ± 4.9 vs 13.5 ± 4.2 μmol/L, p <0.001). In multiple logistic regression models, hyperhomocysteinemia was an independent risk factor for CIN (per the SD change in the plasma homocysteine level [4.44 μmol/L], odds ratio 1.70, 95% confidence interval 1.07 to 2.71, p = 0.025) after adjusting for major risk factors such as age, diabetes, and baseline cardiac and renal function. In subgroup analyses according to diabetes, acute coronary syndrome, or baseline estimated glomerular filtration rate, significant, graded associations were found between the homocysteine level and the incidence of CIN. In conclusion, hyperhomocysteinemia is independently associated with a greater risk of CIN in patients undergoing percutaneous coronary intervention.
Contrast-induced nephropathy (CIN) is one of the major complications after the administration of iodinated contrast media and is associated with poor long-term clinical outcome. Disturbances in renal hemodynamics, endothelial dysfunction, tubular apoptosis resulting from hypoxic damage or reactive oxygen species, and direct cellular toxicity from the contrast agent have been implicated in the proposed pathogenesis of CIN. In contrast, hyperhomocysteinemia is associated with elevated risks of atherosclerosis and cardiovascular disease. Because endothelial dysfunction and oxidative stress are considered potential mechanisms of vascular damage in hyperhomocysteinemia and also contribute to the development of CIN, we hypothesized that hyperhomocysteinemia would be associated with CIN after contrast agent exposure. To test our hypothesis, we investigated whether hyperhomocysteinemia was independently associated with CIN in the patients who underwent percutaneous coronary intervention (PCI).
Methods
The demographic and laboratory data were retrieved from the Severance Cardiovascular Registry Database. A total of 987 patients who underwent PCI at Severance Cardiovascular Hospital from January 2009 to December 2009 were initially included in the present study. The patients who underwent peripheral arterial angioplasty simultaneous with PCI and who were administrated contrast media within 2 weeks before PCI were excluded. In addition, we excluded patients for whom serum homocysteine levels were not available and those with end-stage renal failure requiring dialysis. The final study population consisted of 572 patients. The laboratory parameters such as hemoglobin, serum albumin, blood urea nitrogen, triglycerides, cholesterol, and glucose were measured at PCI using an Advia 2120 Hematology Analyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois). Plasma homocysteine levels were measured using a Hitachi 7600 Analyzer (Hitachi, Tokyo, Japan). The glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation. CIN was defined as an absolute ≥0.5 mg/dl or a relative ≥25% increase in the serum creatinine level at 48 hours after the procedure, which was the most commonly used definition. Standard echocardiography analysis included 2-dimensional, M-mode, and Doppler flow measurements, using a SONOS 7500 (Philips Ultrasound, Bothell, Washington). The institutional review board of Yonsei University Health System Clinical Trial Center approved the study protocol.
Coronary angiography and PCI were performed using the femoral approach according to standard clinical practice. Emergency PCI was performed for patients with ST-segment elevation myocardial infarction. Nonionic, dimeric, iso-osmolar contrast medium (iodixanol; Visipaque, GE Healthcare, Amersham, United Kingdom) was used to visualize the coronary arteries. The patients were hydrated with intravenous normal saline (1.0 to 1.5 ml/kg/hour) for 6 to 12 hours before elective PCI. After the procedure, the hydration was continued for 24 hours at a rate of 1.0 ml/kg/hour, which was decreased to 0.5 ml/kg/hour for patients with volume overload status or left ventricular systolic dysfunction (ejection fraction ≤40%).
Statistical analysis was performed using the Statistical Package for Social Sciences, version 17.0 (SPSS, Chicago, Illinois). Continuous data are expressed as the mean ± SD, and the categorical data are expressed as numbers (percentages). The groups were compared using the analysis of variance for continuous variables or the chi-square test for categorical variables. Multiple logistic regression models were used to analyze the association between plasma homocysteine level and the occurrence of CIN, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. We report the ORs for CIN per increase of 1 SD for all continuous variables in the regression models. The variables identified on univariate analysis as significant predictors of CIN with p <0.05 and those identified as independent predictors in previous reports were included in a multivariate model. The ability of the homocysteine level to predict CIN was analyzed using receiver operating characteristic curve analysis with the calculated area under the receiver operating characteristic curve. p Values <0.05 were considered statistically significant.
Results
The baseline characteristics of the patients according to the tertile of plasma homocysteine level are listed in Table 1 . Patients with higher homocysteine levels were older and predominantly male (p <0.05). In addition, the serum blood urea nitrogen and creatinine levels were significantly greater, and the estimated glomerular filtration rate (eGFR) was significantly lower in the third tertile (p <0.001). However, no differences were found in any other biochemical parameters, co-morbidities, echocardiographic variables, or procedural characteristics among the groups.
| Variable | First Tertile (n = 191) | Second Tertile (n = 191) | Third Tertile (n = 190) | p Value |
|---|---|---|---|---|
| Age (years) | 64 ± 11 | 66 ± 11 | 67 ± 12 | 0.026 |
| Men | 112 (59%) | 141 (74%) | 144 (76%) | <0.001 |
| Body mass index (kg/m 2 ) | 24.5 ± 3.3 | 24.6 ± 3.3 | 24.6 ± 3.6 | 0.90 |
| Systolic blood pressure (mm Hg) | 122 ± 17 | 121 ± 16 | 121 ± 20 | 0.83 |
| Diastolic blood pressure (mm Hg) | 74 ± 11 | 74 ± 12 | 73 ± 13 | 0.32 |
| Smoker | 58 (30%) | 64 (34%) | 80 (42%) | 0.05 |
| Diabetes mellitus | 66 (35%) | 64 (34%) | 78 (41%) | 0.25 |
| Hypertension | 118 (62%) | 121 (63%) | 132 (70%) | 0.25 |
| Hypercholesterolemia | 86 (45%) | 89 (47%) | 78 (41%) | 0.56 |
| Previous coronary arterial bypass graft | 4 (2%) | 6 (3%) | 6 (3%) | 0.77 |
| Clinical diagnosis | 0.65 | |||
| Stable angina pectoris | 88 (46%) | 94 (49%) | 81 (43%) | |
| Unstable angina pectoris | 26 (14%) | 30 (16%) | 30 (16%) | |
| Acute myocardial infarction | 77 (40%) | 67 (35%) | 79 (42%) | |
| Homocysteine (μmol/L) | 9.7 ± 1.6 | 13.3 ± 1.0 | 18.7 ± 3.8 | <0.001 |
| Blood urea nitrogen (mg/dl) | 16.1 ± 6.0 | 18.3 ± 6.4 | 21.2 ± 9.6 | <0.001 |
| Creatinine (mg/dl) | 1.0 ± 0.4 | 1.1 ± 0.4 | 1.3 ± 0.4 | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 79 ± 22 | 75 ± 18 | 62 ± 20 | <0.001 |
| Albumin (g/dl) | 4.3 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 0.35 |
| Total cholesterol (mg/dl) | 171 ± 55 | 164 ± 39 | 164 ± 45 | 0.19 |
| Triglycerides (mg/dl) | 148 ± 92 | 138 ± 74 | 144 ± 82 | 0.52 |
| Glucose (mg/dl) | 126 ± 49 | 127 ± 52 | 131 ± 52 | 0.57 |
| Hemoglobin (g/dl) | 13.1 ± 1.8 | 13.3 ± 1.8 | 13.1 ± 2.1 | 0.71 |
| Echocardiography | ||||
| Ejection fraction (%) | 56 ± 14 | 57 ± 13 | 55 ± 14 | 0.20 |
| E/E′ ratio | 13.6 ± 5.3 | 13.6 ± 4.7 | 14.3 ± 6.3 | 0.39 |
| Extent of coronary artery disease | 0.72 | |||
| One-vessel disease | 70 (37%) | 59 (31%) | 61 (32%) | |
| Two-vessel disease | 60 (31%) | 60 (31%) | 59 (31%) | |
| Three-vessel disease | 61 (32%) | 72 (38%) | 70 (37%) | |
| Cardiogenic shock | 9 (5%) | 8 (4%) | 16 (8%) | 0.16 |
| Use of intra-aortic balloon pump | 8 (4%) | 6 (3%) | 16 (8%) | 0.05 |
| Mean contrast volume administrated (ml) | 217 ± 61 | 225 ± 65 | 226 ± 64 | 0.38 |
| Contrast medium volume ≥300 ml | 22 (14%) | 36 (22%) | 31 (20%) | 0.15 |
Of the 572 patients, 69 (12.1%) developed CIN after PCI. The incidence of CIN was significantly greater in patients in the third homocysteine tertile (CIN rates in the 3 homocysteine groups from lowest to highest, 4.7%, 7.3%, and 24.2%, p <0.001). The patients with CIN were significantly older and had a greater prevalence of diabetes compared to those without CIN. Acute myocardial infarction, 3-vessel coronary artery disease, and the use of intra-aortic balloon pump were more prevalent in patients with CIN. The serum blood urea nitrogen, creatinine, and glucose levels and plasma homocysteine (16.9 ± 4.9 vs 13.5 ± 4.2 μmol/L, p <0.001) were significantly greater in patients with CIN. However, the eGFR, serum albumin and hemoglobin levels, and ejection fraction were significantly lower in patients with CIN. Although the mean amount of contrast dye was not significantly different between the 2 groups, more patients in the CIN group tended to be exposed to a contrast volume ≥300 ml than those without CIN (27% vs 18%, p = 0.15; Table 2 ). Additionally, no significant difference was found in the total amount of fluids until 24 hours after PCI between patients with and without CIN (2,189 ± 357 vs 2,165 ± 426 ml, p = 0.65).
| Variable | CIN | p Value | |
|---|---|---|---|
| No (n = 503) | Yes (n = 69) | ||
| Age (years) | 65 ± 11 | 71 ± 11 | <0.001 |
| Men | 354 (70%) | 43 (62%) | 0.17 |
| Body mass index (kg/m 2 ) | 24.7 ± 3.4 | 23.5 ± 3.2 | 0.007 |
| Systolic blood pressure (mmHg) | 121 ± 17 | 121 ± 24 | 0.92 |
| Diastolic blood pressure (mmHg) | 74 ± 11 | 71 ± 16 | 0.08 |
| Smoker | 174 (35%) | 28 (41%) | 0.33 |
| Diabetes mellitus | 159 (32%) | 49 (71%) | <0.001 |
| Hypertension | 319 (63%) | 52 (75%) | 0.05 |
| Hypercholesterolemia | 224 (45%) | 29 (43%) | 0.77 |
| Previous coronary arterial bypass graft | 9 (2%) | 7 (10%) | <0.001 |
| Clinical diagnosis | <0.001 | ||
| Stable angina pectoris | 251 (50%) | 12 (17%) | |
| Unstable angina pectoris | 75 (15%) | 11 (16%) | |
| Acute myocardial infarction | 177 (35%) | 46 (67%) | |
| Homocysteine (μmol/L) | 13.5 ± 4.2 | 16.9 ± 4.9 | <0.001 |
| Blood urea nitrogen (mg/dl) | 17.5 ± 6.6 | 25.8 ± 11.1 | <0.001 |
| Creatinine (mg/dl) | 1.1 ± 0.3 | 1.6 ±0.7 | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73m 2 ) | 75 ± 19 | 47 ± 19 | <0.001 |
| Albumin (g/dl) | 4.3 ± 0.5 | 3.9 ± 0.6 | <0.001 |
| Total cholesterol (mg/dl) | 166 ± 46 | 167 ± 53 | 0.89 |
| Triglycerides (mg/dl) | 145 ± 82 | 131 ± 88 | 0.23 |
| Glucose (mg/dl) | 125 ± 47 | 151 ± 69 | 0.003 |
| Hemoglobin (g/dl) | 13.4 ± 1.8 | 11.4 ± 2.1 | <0.001 |
| Echocardiography | |||
| Ejection fraction (%) | 57 ± 13 | 46 ± 13 | <0.001 |
| E/E′ ratio | 13.2 ± 4.8 | 18.5 ± 7.3 | <0.001 |
| Extent of coronary artery disease | <0.001 | ||
| One-vessel disease | 180 (36%) | 10 (15%) | |
| Two-vessel disease | 168 (33%) | 11 (16%) | |
| Three-vessel disease | 155 (31%) | 48 (70%) | |
| Cardiogenic shock | 15 (3%) | 18 (26%) | <0.001 |
| Use of intra-aortic balloon pump | 11 (2%) | 19 (28%) | <0.001 |
| Mean contrast volume administrated (ml) | 222 ± 63 | 227 ± 67 | 0.58 |
| Contrast medium volume ≥300 ml | 76 (18%) | 13 (27%) | 0.15 |
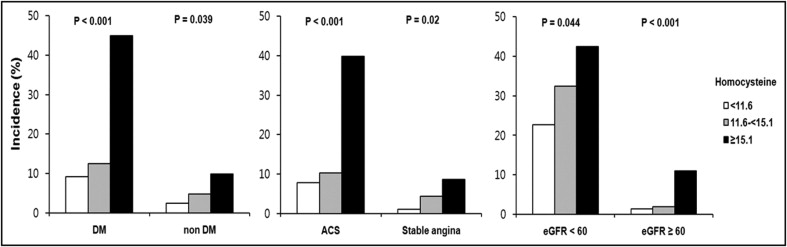
In the subgroup analysis according to diabetes, acute coronary syndrome, or baseline eGFR, significant, graded associations were found between the homocysteine levels and the incidence of CIN in both groups stratified by diabetes, acute coronary syndrome, or eGFR (p <0.05; Figure 1 ) .
In a univariate model using homocysteine as a continuous variable, there was a significant positive association between homocysteine level and the incidence of CIN. Age, diabetes, hyperglycemia, the use of intra-aortic balloon pump, acute myocardial infarction, 3-vessel coronary artery disease, and left ventricular filling pressure were also positively associated with the development of CIN. In addition, decreased renal function, anemia, hypoalbuminemia, and left ventricular systolic dysfunction were associated with CIN. Multivariate logistic regression analysis revealed that baseline homocysteine level (per 1 SD change in plasma homocysteine level [4.44 μmol/L], OR 1.70, 95% CI 1.07 to 2.71, p = 0.025), the presence of diabetes (OR 3.34, 95% CI 1.10 to 10.17, p = 0.034), eGFR level (per 1 SD change in eGFR [21.2 ml/min/1.73m , OR 0.41, 95% CI 0.22 to 0.77, p = 0.005], and the use of an intra-aortic balloon pump (OR 8.63, 95% CI 1.67 to 44.72, p = 0.01) were independent risk factors for the development of CIN after adjusting for the demographic, laboratory, and other echocardiographic parameters ( Table 3 ). Although greater amounts of contrast agent tended to be associated with the development of CIN in the multivariate regression model, the relation did not reach statistical significance. On receiver operating characteristic curve analysis, the homocysteine level was an accurate predictor for the development of CIN; the area under the curve was 0.73 for the baseline homocysteine level (95% CI 0.66 to 0.79, p <0.001; Figure 2 ) .



