Evidence on the usefulness of fibrinogen for the risk stratification of patients with coronary artery disease remains inconclusive. The aims of this study were to investigate the association of fibrinogen with cardiovascular events and to assess whether this biomarker provides additional prognostic information on top of that provided by traditional cardiovascular risk factors. This study included 13,195 patients with angiography-proved coronary artery disease and fibrinogen measurements available. Receiver-operating characteristic curve analysis showed that the best fibrinogen cutoff for mortality prediction was 402.0 mg/dl. On the basis of this cutoff, patients were divided into 2 groups: the group with fibrinogen >402.0 mg/dl (n = 5,198) and the group with fibrinogen ≤402.0 mg/dl (n = 7,997). The primary outcome was 1-year mortality. All-cause deaths occurred in 393 patients with fibrinogen >402.0 mg/dl and in 246 patients with fibrinogen ≤402.0 mg/dl (Kaplan-Meier estimates of mortality 7.7% and 3.1%, log-rank test p <0.001). The relation between fibrinogen and mortality followed a J-shaped pattern, with lowest mortality in patients with fibrinogen concentrations of 295 to 369 mg/dl. After adjustment for cardiovascular risk factors and relevant clinical variables, fibrinogen remained an independent correlate of all-cause mortality (adjusted hazard ratio 1.07, 95% confidence interval 1.04 to 1.10, p <0.001, for each 50 mg/dl increase in fibrinogen level), but it did not improve the discriminatory power of the model for mortality prediction (integrated discrimination improvement 0.002, p = 0.32). In conclusion, in patients with coronary artery disease, fibrinogen is an independent correlate of mortality, but it does not provide additional prognostic information on top of that provided by traditional cardiovascular risk factors.
Numerous previous studies, recently summarized in several meta-analyses, have demonstrated that plasma fibrinogen shows a moderately strong association with the risk for coronary artery disease (CAD), stroke, and vascular and nonvascular mortality. Many previous studies, however, have included subjects or patients who are not representative of contemporary Western populations. More recent studies have shown either that the association between fibrinogen and cardiovascular events lost significance when adjusted for cardiovascular risk factors or that fibrinogen did not provide additional information to that provided by traditional risk factors. Genetic studies and meta-analyses of studies involving β-fibrinogen genotypes suggest that genotypes that produce lifelong differences in fibrinogen concentration are not a major determinant of CAD risk. For these reasons, the National Academy of Clinical Biochemistry stated in its recent laboratory medicine practice guidelines that evidence for the usefulness of fibrinogen in the risk stratification of patients at risk for cardiovascular events is inconclusive. Moreover, other aspects of the association between fibrinogen and cardiovascular events, such as the pattern of fibrinogen-mortality relation or the strength of the association in various subgroups of patients with CAD, remain poorly investigated. We undertook this study with a double objective: (1) to investigate the association between fibrinogen and cardiovascular events in patients with CAD and whether this information is additive to that provided by traditional cardiovascular risk factors and (2) to assess the strength of the association between fibrinogen and mortality in various subgroups of patients.
Methods
This study included 13,195 patients with angiography-proved CAD who underwent percutaneous coronary intervention (PCI) in the German Heart Center in Munich from March 2000 to December 2009. To be included in the study, patients had to have significant CAD, confirmed by coronary angiography at the time of index hospitalization. Patients who underwent PCI in the setting of failed thrombolysis or routine PCI after thrombolysis, as well as those with no fibrinogen measurements available, acute infections, serum creatinine ≥2 mg/dl, and known malignant diseases with life expectancy <1 year, were excluded. All patients gave written informed consent for angiographic examination, performance of PCI, and blood sampling. The study was conducted according to the principles of the Declaration of Helsinki and approved by the institutional ethics committee.
Stable CAD was diagnosed if patients presented with chest pain that did not change its pattern within the past 2 months and had angiographic confirmation of CAD. Acute coronary syndromes were diagnosed using pattern-specific criteria. In all cases, the diagnosis was confirmed by angiographic criteria, which included the presence of coronary stenoses ≥50% luminal obstruction in ≥1 of the 3 major coronary arteries and/or documentation of culprit lesions in case of acute coronary syndromes. The left ventricular ejection fraction was calculated using the area-length method using left ventricular angiography.
Main cardiovascular risk factors were defined using standard criteria. Hypercholesterolemia was defined as a documented total cholesterol value ≥220 mg/dl or previous or ongoing treatment with lipid-lowering agents. Arterial hypertension was defined when a patient was under active treatment with antihypertensive drugs or if the systolic blood pressure was ≥140 mm Hg or the diastolic blood pressure was ≥90 mm Hg on ≥2 separate occasions. Current smokers were those with regular smoking in the previous 6 months. The diagnosis of diabetes required active treatment with insulin or an oral hypoglycemic agent, abnormal fasting blood glucose (≥126 mg/dl), blood glucose >200 mg/dl at any time, or abnormal glucose tolerance according to World Health Organization criteria. Weight and height were measured during the index hospitalization, and body mass index was calculated. The glomerular filtration rate was estimated using the Cockcroft-Gault equation.
PCI and periprocedural care were performed according to standard criteria. Antithrombotic therapy included clopidogrel (300 or 600 mg as a loading dose followed by 75 mg/day for ≥1 month) and aspirin (200 mg/day administered orally and continued indefinitely).
Blood samples were obtained before angiography. Venous blood was collected using the S-Monovettes blood collection system (Sarstedt, Sarstedt, Germany) containing 0.106 mol/L sodium citrate. Nine volumes of blood were mixed with 1 volume of sodium citrate solution. Plasma was separated after immediate centrifugation at 1,500g for 10 minutes. Fibrinogen was determined in citrated plasma by a modification of the Clauss method using the BCS analyzer (Multifibren U; Siemens Healthcare, Erlangen, Germany). The measurement range lies between 80 and 1,200 mg/dl. Expected values range from 180 to 350 mg/dl. Heparin concentration in plasma <2 U/ml does not affect the test. Creatinine was measured using a kinetic colorimetric assay on the basis of the compensated Jaffe method. Laboratory personnel were unaware of clinical, angiographic, or follow-up information.
The primary outcome was all-cause mortality at 1 year after PCI. Secondary outcomes included 1-year occurrences of cardiac mortality, nonfatal myocardial infarction, and stroke. Information on mortality was obtained from hospital records, death certificates, or phone contact with relatives of the patients or referring physicians. Cardiac death was defined according to Academic Research Consortium criteria. The diagnosis of myocardial infarction required the development of new abnormal Q waves in ≥2 contiguous precordial or ≥2 adjacent limb leads or an elevation of creatine kinase-MB >2 times (>3 times for the 48 hours after a PCI procedure) the upper limit of normal. Definite stent thrombosis was defined according to the Academic Research Consortium criteria. Stroke required the occurrence of acute neurologic deficits that were confirmed by computed tomography or magnetic resonance imaging of the head. The follow-up protocol after discharge included a phone interview at 1 month, a visit at 6 months, and a phone interview at 12 months. Follow-up information and adjudication of adverse events was performed by medical staff members unaware of clinical diagnosis or fibrinogen level.
Data are presented as median (interquartile range [IQR]), number of patients or events, or proportions. The Kolmogorov-Smirnov test was used to assess the normality of data distribution. Continuous data were compared using Kruskal-Wallis rank-sum test. Categorical data were compared using chi-square test. Receiver-operating characteristic curve analysis was performed to determine the best fibrinogen cut-off value for the prediction of all-cause mortality while maximizing sensitivity and specificity. Survival analysis was performed using the Kaplan-Meier method, and differences in survival were compared using the log-rank test. The association between fibrinogen and mortality (all-cause or cardiac) was tested using the multivariate Cox proportional-hazards model. All variables listed in Table 1 were entered into the model. The discriminatory power of the multivariate model regarding prediction of mortality was assessed by calculating the integrated discrimination improvement (IDI) according to Pencina et al. Differences in the association between fibrinogen and survival in various subgroups of patients (obtained by dichotomization of patients according to age [cutoff 65 years], gender, diabetes, arterial hypertension, smoking status, hypercholesterolemia, clinical presentation [stable CAD vs acute coronary syndromes], glomerular filtration rate [cutoff 60 ml/min], body mass index [cutoff 30 kg/m 2 ], and the left ventricular ejection fraction [cutoff 50%]) were investigated by performing interaction testing. Analyses were performed using S-Plus (Insightful Corporation, Seattle, Washington). Two-tailed p values <0.05 were considered to indicate statistical significance.
| Characteristic | Fibrinogen (mg/dl) | p Value | |
|---|---|---|---|
| ≤402.0 (n = 7,997) | >402.0 (n = 5,198) | ||
| Age (yrs) | 66.2 (58.4–73.2) | 69.7 (61.8–76.4) | <0.001 |
| Women | 1,511 (19%) | 1,574 (30%) | <0.001 |
| Diabetes mellitus | 1,930 (24%) | 1,756 (34%) | <0.001 |
| Receiving insulin therapy | 530 (7%) | 650 (12.5%) | <0.001 |
| Body mass index (kg/m 2 ) | 26.8 (24.6–29.4) | 27.1 (24.7–30.0) | <0.001 |
| Arterial hypertension | 5,490 (69%) | 3,580 (69%) | 0.79 |
| Current smoker | 1,167 (15%) | 911 (18%) | <0.001 |
| Hypercholesterolemia (≥220 mg/dl) | 5,663 (71%) | 3,577 (69%) | 0.014 |
| Previous myocardial infarction | 2,580 (32%) | 1,601 (31%) | 0.08 |
| Previous coronary artery bypass surgery | 1,209 (15%) | 774 (15%) | 0.72 |
| Clinical presentation | <0.001 | ||
| Stable CAD ∗ | 5,219 (65%) | 2,897 (56%) | |
| Acute coronary syndromes | 2,778 (35%) | 2,301 (44%) | |
| Serum creatinine (mg/dl) | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | <0.001 |
| Glomerular filtration rate (ml/min) | 83.3 (64.9–104.0) | 73.2 (53.5–96.6) | <0.001 |
| Fibrinogen (mg/dl) | 324.0 (279.0–358.0) | 483.0 (436.0–552.0) | <0.001 |
| Number of narrowed coronary arteries | <0.001 | ||
| 1 | 1,519 (19%) | 784 (15%) | |
| 2 | 2,168 (27%) | 1,331 (26%) | |
| 3 | 4,310 (54%) | 3,083 (59%) | |
| Multivessel coronary disease | 6,478 (81%) | 4,414 (85%) | <0.001 |
| Left ventricular ejection fraction (%) | 58.0 (49.0–63.0) | 55.0 (44.0–61.0) | <0.001 |
∗ Defined as chest pain that did not change its pattern within the past 2 months with angiographic confirmation of significant CAD.
Results
The receiver-operating characteristic curve analysis showed that fibrinogen predicted 1-year all-cause mortality with an area under the curve of 0.652 (95% confidence interval [CI] 0.628 to 0.677). The best cut-off value of fibrinogen for the prediction of 1-year all-cause mortality, while maximizing sensitivity and specificity, was 402.0 mg/dl. Using this value, patients were divided into 2 groups: those with fibrinogen >402.0 mg/dl (n = 5,198) and those with fibrinogen ≤402.0 mg/dl (n = 7,997).
Baseline characteristics of patients are listed in Table 1 . With the exception of proportions of patients with arterial hypertension, previous myocardial infarctions, and histories of coronary artery bypass surgery, all other variables appeared to differ significantly between patients in the 2 groups. Of note, patients with fibrinogen >402.0 mg/dl showed a markedly more adverse cardiovascular risk profile compared to those with fibrinogen ≤402.0 mg/dl. In patients with 1-, 2-, and 3-vessel disease, median fibrinogen levels were 355.0 mg/dl (IQR 293.0 to 437.0), 364.0 mg/dl (IQR 303.0 to 447.0), and 376.0 mg/dl (IQR 318.0 to 466.0), respectively (p <0.001). Patients with acute coronary syndromes had higher fibrinogen levels than those with stable CAD (median 383.0 mg/dl [IQR 316.0 to 488.0] vs 362.0 mg/dl [IQR 307.0 to 440.0], p <0.001). Coronary stents were implanted in 90.5% of patients (n = 11,938). Drug-eluting stents were used in 9,023 patients (76%). Statins at discharge were prescribed in 12,041 patients (91.2%), β blockers in 12,401 patients (94%), and angiotensin-converting enzyme inhibitors in 12,225 patients (93%).
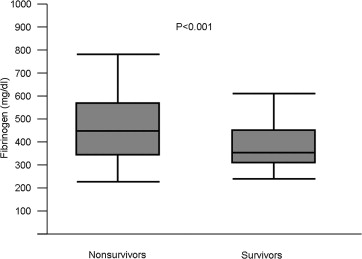
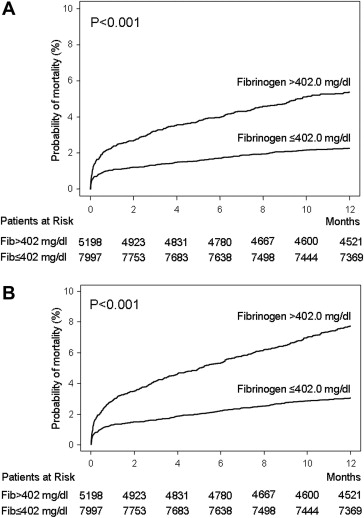
Within the first year after PCI, there were 639 deaths. The median fibrinogen level was 366.0 mg/dl (IQR 308.0 to 450.0) among survivors and 449.0 mg/dl (IQR 347.0 to 576.7) among nonsurvivors (p <0.001; Figure 1 ). All-cause deaths occurred in 393 patients with fibrinogen >402.0 mg/dl and 246 patients with fibrinogen ≤402.0 mg/dl (Kaplan-Meier estimates of mortality 7.7% and 3.1%, respectively, unadjusted hazard ratio [HR] 2.52, 95% CI 2.16 to 2.94, p <0.001; Figure 2 ). Deaths of cardiac origin occurred in 451 patients: 273 among patients with fibrinogen >402.0 mg/dl and 178 among those with fibrinogen ≤402.0 mg/dl (Kaplan-Meier estimates of mortality 5.4% and 2.3%, respectively, unadjusted HR 2.41, 95% CI 2.01 to 2.90, p <0.001; Figure 2 ). Nonfatal myocardial infarctions occurred in 213 patients with fibrinogen >402.0 mg/dl and 204 patients with fibrinogen ≤402.0 mg/dl (Kaplan-Meier estimates of nonfatal myocardial infarction 4.2% and 2.6%, respectively, unadjusted HR 1.63, 95% CI 1.35 to 1.97, p <0.001). Definite stent thrombosis occurred in 50 patients with fibrinogen >402.0 mg/dl and 35 patients with fibrinogen ≤402.0 mg/dl (1.0% vs 0.4%, respectively, unadjusted HR 2.24, 95% CI 1.47 to 3.41, p <0.001). Stroke occurred in 42 patients with fibrinogen >402.0 mg/dl and 51 patients with fibrinogen level ≤402.0 mg/dl (Kaplan-Meier estimates of stroke 0.8% and 0.6%, respectively, unadjusted HR 1.29, 95% CI 0.86 to 1.94, p = 0.22).
The association pattern of fibrinogen with mortality was assessed by analyzing the mortality rates in each decile of fibrinogen concentration ( Table 2 ). The lowest rates of all-cause (2.34% to 2.72%) and cardiac (1.89% to 2.10%) mortality were observed in the third to fifth deciles of fibrinogen (concentration 295 to 369 mg/dl). Mortality increased on both sides of this concentration range, showing a J-shaped fibrinogen-mortality relation ( Figure 3 ).
| Decile of Fibrinogen | Fibrinogen Level (mg/dl) | Number of Patients | Mortality | |
|---|---|---|---|---|
| All-Cause | Cardiac | |||
| 1st | <262 | 1,278 | 49 (3.83) | 35 (2.74) |
| 2nd | 262 to <295 | 1,341 | 40 (2.98) | 28 (2.09) |
| 3rd | 295 to <323 | 1,324 | 31 (2.34) | 25 (1.89) |
| 4th | 323 to <346 | 1,330 | 34 (2.56) | 27 (2.03) |
| 5th | 346 to <369 | 1,288 | 35 (2.72) | 27 (2.10) |
| 6th | 369 to <400 | 1,351 | 53 (2.92) | 34 (2.52) |
| 7th | 400 to <435 | 1,307 | 54 (4.13) | 37 (2.83) |
| 8th | 435 to <482 | 1,324 | 66 (4.98) | 50 (3.78) |
| 9th | 482 to <551 | 1,332 | 96 (7.21) | 64 (4.80) |
| 10th | 551 to 1,190 | 1,320 | 181 (13.71) | 124 (9.39) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


