The association between systolic cardiac dysfunction and arrhythmia development in patients with Duchenne muscular dystrophy (DMD) or Becker muscular dystrophy (BMD) is generally assumed but has not been extensively studied. The purpose of this study was to describe arrhythmias in patients with DMD and BMD in the present era and determine whether arrhythmia development is associated with cardiac dysfunction. This is a single-center retrospective review of 237 Holters from 91 patients with DMD (mean = 17 ± 4 years, range 3 to 27 years) and 64 Holters from 21 patients with BMD (mean = 18 ± 7 years, range 4 to 31 years) with corresponding echocardiography. Holters were stratified by age of patient at the time of study and ejection fraction: normal (≥55%), mild (<55% and ≥45%), moderate (<45% and ≥30%), and severe (<30%). Arrhythmias included frequent atrial and ventricular premature complexes (>10/hr), couplets, and runs of supraventricular and ventricular tachycardias. Arrhythmias occurred in 44% of DMD and 57% of BMD patients and were significantly associated with decrease in cardiac function. Clinically significant arrhythmias (supraventricular tachycardia and ventricular tachycardia) occurred in 10% of all Holters obtained in patients with DMD and 25% of all Holters obtained in patients with BMD. Subgroup analysis of Holters from patients with DMD demonstrated that arrhythmias increased with decreasing ejection fraction regardless of age, but that age was also a significant predictor of arrhythmia development. In conclusion, among patients with DMD or BMD, arrhythmias increase with development of cardiac dysfunction.
Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are both X-linked recessive disorders caused by mutations in the dystrophin gene, with an incidence of 1 in 5,000 and 1 in 19,000 male births, respectively. Clinically, boys affected by DMD first show signs of difficulty from age 2 to 4 years with progression to full-time wheelchair use by 10 to 12 years of age and insidious development of cardiomyopathy and respiratory failure during the next decade of life. In BMD, the progression of disease and the temporal relation between skeletal and cardiac dysfunction are much less predictable because dystrophy and cardiomyopathy may develop at any age. Over the past several decades, increased ventilatory support and early pharmacologic intervention have significantly extended the lifespan of these patients such that a child born with DMD may expect to live well into his third and fourth decades. As these patients are living longer with improved respiratory support, cardiomyopathy has emerged as a significant contributor to morbidity and mortality in this population. In 2010, a study from our center showed that dilated cardiomyopathy in patients with muscular dystrophy is associated with cardiac arrhythmias and electrocardiography abnormalities that frequently precede cardiac dysfunction by several years. However, the association between arrhythmia development and cardiac dysfunction was not well defined. The purpose of this study was to comprehensively describe the rhythm abnormalities found in patients with DMD or BMD using data from Holter recordings and test the hypothesis that arrhythmia development is associated with cardiac dysfunction as measured by echocardiography.
Methods
A retrospective chart review of all patients with DMD or BMD at Texas Children’s Hospital (Houston, Texas) was performed with approval from the internal review board. Patients with both 24-hour Holter monitoring and echocardiography were included. Patients with DMD or BMD generally have a slow decrease in cardiac function, but can also demonstrate acute decrease with subsequent improvement in function, particularly during times of acute respiratory illness. To accommodate the fluctuating ejection fractions (EFs) and associated arrhythmias at the time of function change, each Holter was grouped based on EF from a corresponding echocardiogram and records were reviewed for acute progression of EF decrease as well as acute respiratory decompensation. Holters obtained during routine follow-up were included only if the corresponding echocardiogram was performed within 6 months in patients with normal systolic function and no history of cardiac dysfunction and within 2 months in patients with a history of cardiac dysfunction. Patients with acute respiratory illness or decompensation were included only if an echocardiogram was obtained within 7 days of the Holter. Of the 237 DMD and 64 BMD Holters, 77% and 80% had a corresponding echocardiogram that was obtained within 24 hours of the Holter recording, respectively. If multiple echocardiograms were performed, the one closest in time to the Holter was chosen. Data collection included demographics, clinical history, and detailed review of 24-hour Holter monitoring and echocardiograms.
Holters with associated EF ≥55% were placed in the normal group, EF <55 and ≥45% in the mild dysfunction group, EF <45 and ≥30% in the moderate dysfunction group, and EF <30% in the severe dysfunction group. For purposes of this study, and to document any and all arrhythmias, both clinically significant arrhythmias, defined as supraventricular tachycardia (SVT) or ventricular tachycardia (VT), and lower grade rhythm disturbances such as frequent ectopy or couplets were recorded. Arrhythmias were therefore defined as (1) frequent isolated atrial (APC) or ventricular (VPC) premature complexes, >10/hour, (2) atrial or ventricular couplets, and (3) nonsustained (at least 3 beats and <30 seconds) or sustained (≥30 seconds) SVT and VT, excluding sinus tachycardia.
For trend analysis, the extended Mantel–Haenszel chi-square test was used. Owing to multiple Holter recordings on some of the same patients (repeated measures), generalized estimating equations with binomial distribution and logit link function were applied to estimate the odds ratio (OR) and p values for final EF and age between patients with and without arrhythmias. SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina) was used for all data analysis. Data are presented as mean ± SD for continuous variables and number with percentage for categorical variables. A p value <0.05 was considered statistically significant. For multivariate analysis by subgroup EF, the moderate and severe groups were combined because of small sample numbers. Age at arrhythmia occurrence was initially evaluated as a continuous variable. To determine how age was related to the likelihood of developing arrhythmias, data were plotted and a receiver operating characteristic curve constructed to maximize sensitivity and specificity.
Results
A retrospective chart review was conducted on 266 patients with DMD and 56 patients with BMD at Texas Children’s Hospital based on data from 2008 to 2015. Of these patients, all of whom were males, 91 patients with DMD and 21 patients with BMD had 24-hour Holter recordings with corresponding echocardiography that met study criteria. From these patients, a total of 237 and 64 Holter studies with corresponding echocardiography were reviewed and summarized. At the time of Holter monitoring, patients with DMD ranged in age from 3 to 27 years (mean 17 ± 4 years) with EF ranging from 17% to 68% (mean 49 ± 11%). Patients with BMD ranged in age from 4 to 31 years (mean 18 ± 7 years) with EF ranging from 8% to 71% (mean of 45 ± 14%; Tables 1 and 2 ).
| Groups | Total | Normal (EF≥55%) | Mild (45%≤EF<55%) | Moderate (30%≤EF<45%) | Severe (EF<30%) |
|---|---|---|---|---|---|
| Number of Patients ∗ | 91 | 53 | 41 | 31 | 8 |
| Number of Holters | 237 | 99 | 65 | 58 | 15 |
| Arrhythmias | |||||
| Any arrhythmia | 73(31%) | 18(18%) | 20(31%) | 27(47%) | 8(53%) |
| SVT or VT | 24(10%) | 3(3%) | 4(6%) | 14(24%) | 3(20%) |
| APC ≥ 10/hr | 4(2%) | 0 | 1(2%) | 3(5%) | 0 |
| Atrial couplets | 16(7%) | 7(7%) | 2(3%) | 6(10%) | 1(7%) |
| SVT | 9(4%) | 1(1%) | 1(2%) | 6(10%) | 1(7%) |
| VPC ≥ 10/hr | 34(14%) | 2(2%) | 10(15%) | 16(28%) | 6(40%) |
| Ventricular couplets | 48(20%) | 10(10%) | 16(25%) | 15(26%) | 7(47%) |
| VT | 17(7%) | 2(2%) | 4(6%) | 9(16%) | 2(13%) |
| Anti-beta-adrenergic † | |||||
| Carvedilol (low dose) | 45(19%) | 5(5%) | 13(20%) | 17(29%) | 10(67%) |
| Carvedilol (high dose) | 24(10%) | 4(4%) | 6(9%) | 11(19%) | 3(20%) |
| Metoprolol | 36(15%) | 15(15%) | 11(17%) | 9(16%) | 1(7%) |
| Age at Holter (yr), mean±SD | 17±4 | 15±4 | 17±4 | 19±3 | 18±5 |
| HR (bpm), mean±SD | 95±13 | 97±12 | 95±12 | 93±14 | 95±15 |
| EF (%), mean±SD ‡ | 49±11 | 59±3 | 50±3 | 39±4 | 25±4 |
∗ Some patients are counted in more than one group because they have Holters in multiple groups; percentages reflect percent of Holters, not patients.
† Carvedilol: low dose is anything ≤6.25 mg twice a day; high dose is anything higher. None of the patients were on any other antiarrhythmics such as amiodarone.
‡ Numerical data not available from 20 Holters in the normal group, 10 in the mild group, 18 in the moderate group, and 1 in the severe group.
| Groups | Total | Normal (EF≥55%) | Mild (45%≤EF<55%) | Moderate (30%≤EF<45%) | Severe (EF<30%) |
|---|---|---|---|---|---|
| Number of Patients ∗ | 21 | 14 | 7 | 6 | 3 |
| Number of Holters | 64 | 25 | 10 | 22 | 7 |
| Arrhythmias | |||||
| Any arrhythmia | 40(63%) | 6(24%) | 6(60%) | 21(95%) | 7(100%) |
| SVT or VT | 16(25%) | 1(4%) | 3(30%) | 11(50%) | 1(14%) |
| APC ≥ 10/hr | 3(5%) | 0 | 3(30%) | 0 | 0 |
| Atrial couplets | 17(27%) | 4(16%) | 2(20%) | 8(36%) | 3(43%) |
| SVT | 7(11%) | 1(4%) | 2(20%) | 4(18%) | 0 |
| VPC ≥ 10/hr | 24(38%) | 0 | 3(30%) | 17(77%) | 4(57%) |
| Ventricular couplets | 32(50%) | 2(8%) | 4(40%) | 19(86%) | 7(100%) |
| VT | 13(20%) | 1(4%) | 2(20%) | 9(41%) | 1(14%) |
| Anti-beta-adrenergic † | |||||
| Carvedilol (low dose) | 8(13%) | 1(4%) | 3(30%) | 3(14%) | 1(14%) |
| Carvedilol (high dose) | 21(33%) | 0 | 4(40%) | 15(68%) | 2(29%) |
| Metoprolol | 12(19%) | 3(12%) | 2(20%) | 4(18%) | 3(43%) |
| Age at Holter (yr), mean±SD | 18±7 | 13±5 | 19±5 | 23±5 | 18±8 |
| HR (bpm), mean±SD | 82±12 | 88±9 | 82±14 | 72±9 | 87±7 |
| EF (%), mean±SD ‡ | 45±14 | 61±3 | 47±3 | 37±4 | 19±8 |
∗ Some patients are counted in more than one group because they have Holters in multiple groups; percentages reflect percent of Holters, not patients.
† Carvedilol: low dose is anything ≤6.25 mg twice a day; high dose is anything higher. None of the patients were on any other antiarrhythmics such as amiodarone.
‡ Numerical data not available from 6 Holters in the normal group.
Of the 91 patients with DMD, a total of 40 patients (44%) had some form of arrhythmia, including frequent APC in 3%, frequent VPC in 22%, atrial couplets in 16%, ventricular couplets in 32%, SVT in 9%, and VT in 13% of patients. A total of 73 (31%) Holters revealed some form of arrhythmia including 24 (10%) with nonsustained SVT or VT. Ventricular arrhythmias were more common than supraventricular arrhythmias and ventricular ectopy was more common than VT. A summary of the arrhythmia findings is listed in Table 1 .
Of the 21 patients with BMD, 12 patients (57%) had some form of arrhythmia including frequent APC (5% of all patients), frequent VPC (8%), atrial couplets (13%), ventricular couplets (16%), SVT (6%), and VT (11%). Similar to patients with DMD, ventricular arrhythmias were more common although both supraventricular and ventricular arrhythmias were seen. A total of 40 (63%) Holters demonstrated some form of arrhythmia. A summary of the arrhythmia findings is listed in Table 2 .
To understand whether arrhythmia development was associated with systolic cardiac dysfunction, Holters were stratified based on the corresponding EF (see Methods and Table 1 ). Among patients with DMD we found that (1) arrhythmias occurred even in the setting of normal systolic function and (2) the percentage of Holters with arrhythmias increased with worsening systolic function (p <0.001). Although 18% of Holters demonstrated some form of arrhythmia in patients with normal EF, arrhythmias were seen in 31% with mild dysfunction, 47% with moderate dysfunction, and 53% with severe dysfunction ( Table 1 ). Although the overall trend for arrhythmias increased with worsening function (p <0.001), this trend was significant only for ventricular (p <0.01) and not supraventricular arrhythmias (p = not significant).
To test this association while taking into account repeated measures, generalized estimating equations models were used to evaluate the OR of arrhythmia development as cardiac function declined. First, univariate analysis revealed that arrhythmias were associated with worsening EF; however more specifically, although development of any arrhythmia (including frequent ectopy) increased between all progressive EF stages, clinically significant arrhythmias such as SVT and VT did not occur until systolic function decreased to the moderate or severe range ( Table 3 ).
| Variable | OR ∗ | 95% CI | p-value |
|---|---|---|---|
| Any arrhythmia | |||
| Mild vs. Normal | 2.33 | 1.21-4.49 | 0.011 |
| Moderate vs. Normal | 3.85 | 1.75-8.47 | <0.001 |
| Severe vs. Normal | 5.66 | 1.23-25.96 | 0.026 |
| Age at Holter in Years | 1.17 | 1.05-1.30 | 0.004 |
| SVT/VT | |||
| Mild vs. Normal | 2.37 | 0.59-9.54 | 0.223 |
| Moderate vs. Normal | 9.54 | 2.56-35.56 | <0.001 |
| Severe vs. Normal | 8.13 | 1.40-47.14 | 0.019 |
| Age at Holter in Years | 1.19 | 1.05-1.35 | 0.005 |
∗ For every decrease in EF from normal to mild, moderate, or severe dysfunction, or for every increase in year of age at Holter, the OR estimates the increase in the risk of any arrhythmia or SVT/VT.
Next, multivariate analysis was performed to evaluate whether age was a confounding factor. Table 4 lists that after adjusting for age, arrhythmias remained significantly associated with development of moderate/severe dysfunction but not with progression from normal to mild dysfunction. Furthermore, when evaluated as a continuous variable, age was significantly associated with the development of any arrhythmia (p = 0.029) but neared significance for SVT/VT (p = 0.051). For this reason, to maximize sensitivity and specificity, we further investigated the relation of age and arrhythmia development and dichotomized age into <17 years and ≥17 years (see Methods ). Repeating the multivariate analysis, the development of SVT or VT remained significantly associated with moderate/severe dysfunction (OR = 6.72, p = 0.004) and was also significantly associated with age ≥17 years (OR = 3.30; p = 0.028; Table 5 ). In other words, both the decrease in EF to the moderate/severe ranges and the increase of age ≥17 years were significantly associated with the development of SVT/VT.
| Variable | Adjusted OR ∗ | 95% CI | p-value |
|---|---|---|---|
| Any arrhythmia | |||
| Mild vs. Normal | 1.85 | 0.92-3.71 | 0.085 |
| Moderate/Severe vs. Normal | 3.00 | 1.26-7.17 | 0.013 |
| Age at Holter in Years | 1.13 | 1.01-1.26 | 0.029 |
| SVT/VT | |||
| Mild vs. Normal | 1.80 | 0.42-7.64 | 0.427 |
| Moderate/Severe vs. Normal | 6.62 | 1.88-23.33 | 0.003 |
| Age at Holter in Years | 1.14 | 1.00-1.29 | 0.051 |
∗ The adjusted OR estimates the increase in the risk of any arrhythmia or SVT/VT for each comparison of EF groups and for each unit change of age at Holter.
| Variable | Adjusted OR ∗ | 95% CI | p-value |
|---|---|---|---|
| Mild vs. Normal | 1.57 | 0.36-6.84 | 0.550 |
| Moderate/Severe vs. Normal | 6.72 | 1.82-24.88 | 0.004 |
| Age at Holter (≥17 yrs vs. <17 yrs) | 3.30 | 1.14-9.60 | 0.028 |
∗ Adjusted OR estimates the increase in the risk of SVT/VT for each comparison of EF groups or age bins.
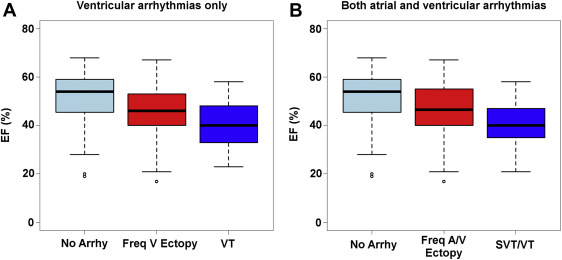
Finally, these data were plotted using EF as a continuous rather than categorical variable. The corresponding EFs of these groups were plotted as box plots in Figure 1 , which demonstrates that arrhythmias can occur even at normal EF ranges but worsening arrhythmias appear to occur with lower EFs. This supports the hypothesis that decreasing EF is associated with increased incidence and severity of arrhythmias.