The aim of this study was to investigate the impact of myocardial fibrosis in Fabry disease. Seventy-three patients with genetically confirmed Fabry disease were followed for 4.8 ± 2.4 years. In accordance with current guidelines, 57 patients received enzyme replacement therapy (ERT) after study inclusion, whereas 16 did not. At baseline and latest possible follow-up, myocardial fibrosis was assessed noninvasively by cardiac magnetic resonance, and biomarkers of collagen metabolism were determined. Holter electrocardiography and clinical follow-up at yearly intervals were used to monitor malignant ventricular arrhythmias (MVAs; nonsustained and sustained ventricular tachycardia and sudden cardiac death). In total, 48 patients (66%) showed fibrosis assessed by late enhancement (LE) at baseline, and 4 patients developed new LE during follow-up, 2 of them despite ERT. The 2 patients receiving ERT (1.4 ± 1.9% vs 2.5 ± 2.6%, p <0.001) and the patients not receiving ERT (0.5 ± 0.8% vs 0.7 ± 1.0%, p = 0.035) showed a progression of LE during follow-up. None of the patients displayed reductions of LE during follow-up. Collagen biomarkers were elevated in patients with and without LE but did not correlate with LE amount. Thirteen LE-positive patients at the baseline examination had documented MVAs (including 5 sudden cardiac deaths), whereas none of the patients without LE had MVAs. The yearly increase in fibrosis was 0.9 ± 0.6% in patients with MVAs and 0.2 ± 0.3% in patients without MVAs (p <0.001). Logistic multivariate regression analysis revealed that the annual increase in fibrosis during follow-up was the only independent predictor of MVAs. In conclusion, myocardial fibrosis in Fabry disease is progressive, apparently not modified by ERT, and a crucial outcome determinant.
Fabry disease is an X-linked lysosomal storage disorder caused by a deficiency of α-galactosidase A. Cardiac involvement is characterized by the accumulation of globotriaosylceramide in cells, causing left ventricular (LV) hypertrophy and finally leading to myocardial replacement fibrosis in the hearts of patients with Fabry disease. The dominant cardiac symptoms are arrhythmias and heart failure, which determine the reduced life expectancy observed in patients with Fabry disease. The reference standard for noninvasively assessing myocardial fibrosis is cardiac magnetic resonance (CMR) using the late enhancement (LE) technique, and various studies have successfully used this tool to investigate myocardial fibrosis in patients with Fabry disease. Although LE was used to stage patients with Fabry disease, a prospective longitudinal study to quantify the development of myocardial fibrosis is lacking, especially in patients receiving enzyme replacement therapy (ERT). Moreover, the prognostic relevance of myocardial fibrosis in Fabry disease needs further evaluation. We hypothesized that the fibrotic progression in the Fabry cardiomyopathy has an impact on prognosis. Thus, we investigated the impact of myocardial fibrosis in this disease using CMR LE imaging and collagen biomarkers and determined the occurrence of malignant ventricular arrhythmias (MVAs).
Methods
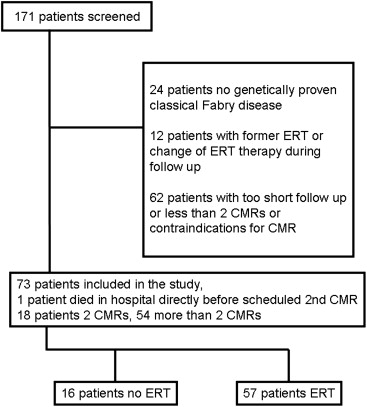
In total, 171 referred patients were screened at their first visits to the Fabry center in Würzburg ( Figure 1 ). All of the following criteria had to be met for inclusion in this observational study: (1) genetically proved Fabry disease, (2) the ability to undergo CMR imaging with contrast agent, (3) no ERT before this study, (4) no ERT therapy switch during follow-up, (5) a minimum follow-up time of 1 year with 2 corresponding CMR scans or death during follow-up, and (6) informed consent. Overall, 73 consecutive patients with Fabry disease (35 female) fulfilled all these criteria and were included. Recruitment started in January 2001 and ended in September 2009. The treatment decision for ERT was reviewed at study start, and in accordance with current guidelines, 57 patients received ERT and 16 did not. In accordance with the Declaration of Helsinki, written informed consent from all patients or their guardians was obtained, and the local ethics board approved the protocol. Several patients from the present study cohort have been included in other open-cohort studies from our center.
CMR was performed at first presentation and latest possible follow-up with intravenous injection of gadobenate dimeglumine 0.1 mmol/kg (MultiHance; Bracco Diagnostics, Milan, Italy) on a 1.5-T scanner (Magnetom Symphony Quantum; Siemens Medical Systems, Erlangen, Germany). Standard steady-state free precession cine breath-hold short-axis 4-, 3-, and 2-chamber images were used to determine wall thickness, cardiac mass, and the ejection fraction. LE images ( Figure 2 ) were acquired with inverse recovery sequences (slice thickness 8 mm, breath hold, field of view 240 × 320 mm 2 , matrix size 165 × 256, repetition time 7.5 ms, echo time 3.4 ms, flip angle 25°). Care was taken to use the same settings for the follow-up scan.

The analysis was done blinded to all clinical data by an experienced CMR specialist (>15 years’ experience in CMR, >10 years’ experience with Fabry disease). Inter- and intraobserver variability was recently published by our group.
All consecutive short-axis slices were used for measuring the area with pathologic LE. The sum of areas was multiplied by the slice thickness and then set in relation to LV myocardial volume (the amount of LV fibrosis as a percentage). To calculate the percentage change in fibrosis over time in a single patient, we subtracted the percentage of fibrosis determined in the last available CMR examination from the result taken from the baseline CMR examination. Any CMR examinations performed between these times points were omitted from the analysis.
Peripheral venous blood samples were obtained after 30 minutes of rest in the supine position at baseline and latest possible follow-up. Blood samples were immediately centrifuged at 4°C at 10g for 10 minutes. Afterward, aliquots of 100 to 250 ml serum were stored at −20°C for ≤3 days until final storage in a freezer at −80°C. All aliquots were analyzed at the end of the study period. Serum procollagen type III aminoterminal propeptide, procollagen type I carboxyterminal propeptide, and collagen type I carboxyterminal telopeptide levels were quantified using a radioimmunoassay technique using commercially available kits from USCN Life Science Inc. (Wuhan, China), as previously described.
The occurrence of MVAs (nonsustained and sustained ventricular tachycardia [VT] and sudden cardiac death) was determined in annual intervals by Holter electrocardiography of ≥24 hours in duration (mean 28 ± 5.5 hours). All available Holter electrocardiograms for each patient were analyzed for the detection of arrhythmias. Sudden cardiac death was defined as an unexpected arrest of presumed cardiac origin within 1 hour after the onset of any symptoms that could be interpreted as being cardiac in origin.
All values are presented as absolute number (percentage) or mean ± SD. Differences between 2 groups were tested using paired or unpaired t tests, chi-square tests, or Fisher’s exact tests, as appropriate. Higher group numbers were tested using 2-way analysis of variance with Duncan’s post hoc analysis and Bonferroni’s correction. A Kaplan-Meier curve was plotted for visualizing the event-free rate of MVAs of patients with and without fibrosis as indicated by LE, and a log-rank test was performed to statistically compare the 2 survival curves. Predictors of MVAs were sought using logistic regression analysis. The multivariate regression analysis included percentage LV fibrosis, LV mass, septal wall thickness, blood pressure, annual increase of LV mass, and annual increase of percentage LV fibrosis, all selected on the basis of univariate predictors of MVAs. The multivariate logistic regression analysis was performed with fixed adjustment for age, gender, kidney function, and ERT status. A p value <0.05 or corrected values in multiple testing were deemed to indicate statistical significance. Data were analyzed using SPSS versions 20 and 21 (SPSS, Inc., Chicago, Illinois).
Results
Seventy-three consecutive patients with Fabry disease were included in this observational study and followed for 4.8 ± 2.4 years. Table 1 lists the general clinical data of the complete cohort, indicating reasonably preserved kidney and global LV function and moderate LV hypertrophy. In total, 48 patients (66%) exhibited fibrosis as assessed by LE on CMR at baseline. Table 2 lists the baseline CMR and biomarker data, split into groups of patients with and without fibrosis. There was no difference in the ejection fraction; however, significant differences were seen in LV wall thickness and LV mass. Collagen biomarkers were elevated in the 2 groups compared with healthy controls reported in the published research but did not differentiate between groups. During follow-up, patients with and those without ERT showed progressive LE, as evidenced by an increase in percentage LV fibrosis on repeat CMR ( Table 3 ). Those with ERT showed an increase from 1.4 ± 1.9% at baseline to 2.5 ± 2.6% at follow-up (p <0.001); those without ERT showed an increase from 0.5 ± 0.8% to 0.7 ± 1.0% (p = 0.035). None of the patients showed reductions in LE at follow-up. Four patients developed new fibroses at rates of +0.9% after 8 years (no ERT), +0.7% after 6 years (no ERT), +1.7% after 7 years (ERT), and +1.0% after 5 years (ERT). There was no significant change in any of the biomarkers in either the ERT-naive or in the ERT group. Results are listed according to ERT status in Table 3 .
| Male/Female | 38/35 (48%/52%) |
| Age (years) | 39 ± 11 |
| Height (cm) | 172 ± 10 |
| Weight (kg) | 68 ± 13 |
| Heart rate (1/min) | 66 ± 14 |
| Systolic blood pressure (mmHg) | 123 ± 18 |
| Diastolic blood pressure (mmHg) | 81 ± 12 |
| Abnormal sweating | 50 (67%) |
| Heat or cold intolerance | 49 (67%) |
| Chronic diarrhea | 30 (41%) |
| Sudden deafness | 18 (25%) |
| Angiokeratomata | 30 (41%) |
| Dialysis | 7 (10%) |
| Kidney transplantation | 6 (8%) |
| Serum creatinine (mg/dl) | 1.5 ± 2.1 |
| Diethylene triamine pentaacetic acid clearance (ml/min) | 93 ± 32 |
| Stroke/transitory ischemic attack | 9 (12%) |
| Typical neuropathic pain | 51 (79%) |
| Chronic pain syndrome | 32 (44%) |
| Depression | 11 (15%) |
| Left-ventricular mass (g/m 2 ) | 85 ± 32 |
| Left-ventricular ejection fraction (%) | 63 ± 8 |
| Septal wall thickness (mm) | 11.3 ± 3.8 |
| Amount of fibrosis (% of left-ventricular mass) | 1.2 ± 1.7 |
| No Fibrosis (n = 25) | Fibrosis (n = 48) | |
|---|---|---|
| Left-ventricular mass (g/m 2 ) | 70 ± 16 | 93 ± 36 ∗ |
| Septal wall thickness (mm) | 9.4 ± 2.4 | 12.2 ± 4.0 ∗ |
| Ejection fraction (%) | 62 ± 7 | 64 ± 9 |
| Amount of fibrosis (% of left-ventricular mass) | 0 | 1.8 ± 1.8 ∗ |
| Procollagen type I carboxy-terminal propeptide (ng/ml) | 308 ± 399 | 302 ± 361 |
| Collagen type I carboxy-terminal telopeptide (ng/ml) | 8.3 ± 15.3 | 8.0 ± 12.9 |
| Procollagen type III amino-terminal propeptide (μg/l) | 5.9 ± 2.4 | 6.8 ± 3.7 |
| Malignant ventricular arrhythmias | 0 (0%) | 13 (27%) |
| Sudden cardiac death | 0 (0%) | 5 (10%) |
| No Enzyme Replacement Therapy; | Enzyme Replacement Therapy; | |||
|---|---|---|---|---|
| Baseline (n = 16) | Follow-Up (n = 16) | Baseline (n = 57) | Follow-Up (n = 56) ∗ | |
| Left-ventricular mass (g/m 2 ) | 59 ± 11 | 61 ± 14 | 92 ± 33 † | 90 ± 30 ‡ |
| Septal wall thickness (mm) | 8.0 ± 1.6 | 8.6 ± 1.9 | 12.2 ± 3.7 † | 12.3 ± 3.7 ‡ |
| Ejection fraction (%) | 60 ± 6 | 59 ± 8 | 64 ± 9 | 63 ± 9 |
| Amount of fibrosis (% of left-ventricular mass) | 0.5 ± 0.8 | 0.7 ± 1.0 § | 1.4 ± 1.9 † | 2.5 ± 2.6 ‡ § |
| Procollagen type I carboxy-terminal propeptide (ng/ml) | 319 ± 362 | 194 ± 290 | 299 ± 377 | 240 ± 346 |
| Collagen type I carboxy-terminal telopeptide (ng/ml) | 9.9 ± 18.4 | 6.2 ± 14.7 | 6.2 ± 14.7 | 9.8 ± 15.0 |
| Procollagen type III amino-terminal propeptide (μg/l) | 5.7 ± 1.6 | 5.9 ± 1.7 | 5.9 ± 1.7 | 6.8 ± 3.1 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


