Stent thrombosis is a devastating complication after percutaneous coronary intervention (PCI), but the influence of obesity on risk of stent thrombosis is unclear, and it is unknown if this relation is dependent on stent type. The objective of this study was to examine the relation between body mass index (BMI) and stent thrombosis after PCI with bare-metal stent (BMS) or drug-eluting stent (DES). We followed 5,515 patients who underwent PCI with implantation of ≥1 BMS or DES at a high-volume tertiary invasive cardiology center from 2000 through 2006. Only patients with a single type of stent (BMS or DES) implanted at the index PCI were included. Median follow-up period was 26 months (interquartile range 12 to 44) and definite stent thrombosis occurred in 78 patients. Hazard ratio of definite stent thrombosis adjusted for number of stents at the index PCI was 0.92 (95% confidence interval [CI] 0.86 to 0.97) for each increase in kilograms per square meter of BMI. There was no significant interaction between stent type and BMI (p = 0.48). Hazard ratios for probable stent thrombosis and possible stent thrombosis adjusted for numbers of stents at the index PCI were 1.01 (CI 0.99 to 1.03) and 0.99 (CI 0.98 to 1.01) for each increase in kilograms per square meter of BMI, respectively. In conclusion, BMI was inversely correlated with risk of definite stent thrombosis after PCI irrespective of stent type.
The impact of body mass index (BMI) on prognosis after percutaneous coronary intervention (PCI) has been the subject of several studies and most of these studies have suggested the existence of an obesity paradox, i.e., that despite being associated with increased risk of coronary artery disease, increased BMI predicts a more favorable outcome after PCI. Although such studies generally have focused on mortality and a composite of major adverse cardiac events, there is limited and contradictory evidence available on the impact of obesity on risk of stent thrombosis after PCI, including uncertainty about whether such relation is dependent on stent type, i.e., bare-metal stent (BMS) versus drug-eluting stent (DES). Data in this area of research are furthermore limited with regard to outcomes beyond 1 year after PCI, and debate remains on whether DESs (irrespective of the BMI of the patient) are associated with higher rates of late stent thrombosis than BMSs. We therefore investigated the impact of BMI on the medium- to long-term risk of stent thrombosis with stent thrombosis as the primary end point after PCI with BMS or DES.
Methods
We used a dedicated catheterization laboratory database to identify 6,079 consecutive patients who underwent successful PCI with implantation of ≥1 stent at the PCI laboratory of Gentofte University Hospital, Copenhagen, Denmark from January 1, 2000 to December 31, 2006. The institution is a highly specialized tertiary care invasive cardiology center where high-volume operators (each >300 procedures/year) provide PCI for the population of the northern part of the Zeeland region in Denmark, including 24-hour primary PCI with a catchment area of >1 million residents, i.e., 20% of the Danish population. The database holds information on baseline characteristics and procedural details of all patients who underwent PCI at this institution during the study period, with the operator filling out all information. The single-study inclusion criterion for the present analysis was successful PCI as determined by the operator, with implantation of BMSs or DESs and residual stenosis <20% of the target vessel diameter after PCI. The national Danish health care organization and reimbursement system encourage that patients in the catchment area who develop acute high-risk coronary syndromes after previous PCI be transferred for coronary angiography and revascularization to the institution that performed the index procedure. Validation at a national level by use of the Danish National Patient Registry confirmed that 99% of patients with previous PCI who later presented with acute coronary syndromes were transferred for coronary angiography and revascularization to the hospital that performed the index PCI (not shown).
We defined urgency of index PCI as follows: ST-segment elevation myocardial infarction (MI) as acute, non–ST-segment elevation MI or unstable angina pectoris as subacute, and all other discharge diagnoses, primarily stable angina pectoris, as elective.
In Denmark every citizen is assigned a personal and permanent identification number at birth or immigration, which makes cross linkage of different registries at an individual level possible. We obtained data on vital status from the Danish Central Population Register, in which all deaths are recorded within 2 weeks. Causes of death were obtained from the National Causes of Death Register in which all diagnoses since 1995 are coded according to the International Classification of Diseases, Tenth Revision . From the Danish National Patient Register we obtained detailed information on all hospitalizations. Each hospitalization is registered with 1 primary and, if appropriate, ≥1 secondary diagnosis according to the International Classification of Diseases, Tenth Revision . Data on pharmacotherapy at discharge after the index PCI were obtained from the Register of Medicinal Product Statistics, which holds complete information of all dispensed drug prescriptions from Danish pharmacies since January 1, 1995. This register is directly linked to the system for reimbursement of drug expenses and has been shown to be accurate. Drugs were registered according to the international Anatomical Therapeutical Chemical classification system. Treatment after discharge with statins (C10A), β blockers (C07), warfarin (B01AA0), angiotensin-converting enzyme inhibitors (C09AA), angiotensin II receptor blockers (C09CA), digoxin (C01AA05), and aspirin (B01AC06) were ascertained, as was treatment with clopidogrel (B01AC04). In Denmark aspirin can be bought as an over-the-counter drug and therefore is not consecutively registered in the Register of Medicinal Product Statistics for all patients. We therefore used dispensed prescriptions for clopidogrel as a surrogate for use of dual-antiplatelet therapy. Diabetes was defined as use of glucose-lowering medications (A10) within 90 days after discharge. Hypertension was defined as treatment with ≥2 different types of antihypertensive agents, which has been validated with a specificity of 94.7% and a positive predictive value of 80.0%. BMI was calculated by dividing the registered weight in kilograms with the squared height in meters at baseline, and patients were divided into 4 groups according to the World Health Organization classification, i.e., underweight (BMI <18.5 kg/m 2 ), normal weight (BMI 18.5 to 24.9 kg/m 2 ), overweight (BMI 25.0 to 29.9 kg/m 2 ), and obese (BMI ≥30.0 kg/m 2 ). Co-morbidity scores were calculated using the Charlson Co-Morbidity Index, which includes 19 major disease categories.
The primary outcome of stent thrombosis was classified as definite, probable and possible stent thrombosis according to criteria from the Academic Research Consortium with minor modifications. We included only the first stent thrombosis in our analyses, i.e., each patient could have only 1 study end point, resulting in exclusion of 2 definite stent thrombosis events and 9 probable stent thrombosis events that occurred in patients who already had 1 stent thrombosis event during the study period. Definite stent thrombosis was defined as angiographically confirmed thrombosis in the coronary segment with the index stent(s) in the setting of acute MI ( International Classification of Diseases, Tenth Revision code DI21). MI diagnosis in the Danish National Patient Register has previously been validated with a sensitivity of 91% and a positive predictive value of 93%. Probable stent thrombosis was defined as unexplained death or MI without angiographic confirmation of stent thrombosis within the first 30 days. Possible stent thrombosis was defined as unexplained or assumed cardiovascular death after 30 days from the index PCI and not registered as probable stent thrombosis. Secondary outcomes were target lesion revascularization, target vessel revascularization, cardiovascular mortality (DI00 to DI99), and all-cause mortality.
Baseline variables for BMI groups were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. Time-to-event data were analyzed using a Kaplan–Meier estimator and compared using log-rank tests. We used multivariable Cox proportional hazard regression analyses to investigate the impact of BMI on the primary and secondary outcomes. BMI was used primarily as a continuous variable. Group-wise analyses of underweight patients must be interpreted with great caution because a power analysis showed that 564 patients were required in each BMI group to obtain a power of 0.90 with a type I error rate of 0.05 when the reference event rate of stent thrombosis was set to 0.01 and the expected proportional difference was set to 0.05. All analyses were adjusted for number of implanted stents at index PCI. Additional analyses were adjusted for age, gender, year of index PCI, index stent, Charlson Co-Morbidity Index score, diabetes, treatment with statins, treatment with clopidogrel, length and diameter of index stent, and urgency of PCI (i.e., acute vs elective and subacute vs elective). Model assumptions, i.e., proportional hazards, lack of interactions, and linearity of continuous variables, were tested and found to be valid unless otherwise indicated. When the proportional hazard assumption was not valid, the variable was included as a time-dependent variable. All p values were 2-sided and a p value <0.05 was considered statistically significant. All statistical calculations were performed using SAS 9.1 for Windows (SAS Institute, Cary, North Carolina).
Results
Patients without BMI data (n = 301) and those who simultaneously received BMS(s) and DES(s) (n = 263) at the index PCI were excluded from the study, yielding a final study population of 5,515 subjects. Median follow-up period was 26 months (interquartile range 12 to 44). Baseline, angiographic, and PCI procedural characteristics of the study population divided into the 4 BMI groups are presented in Tables 1 and 2 , respectively.
| Variable | BMI (kg/m 2 ) | p Value | |||
|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30.0 | ||
| (n = 60) | (n = 1,991) | (n = 2,399) | (n = 1,065) | ||
| Follow-up time (days) | 471 (60–1,427) | 778 (345–1,322) | 830 (375–1,359) | 764 (366–1,314) | |
| Men | 16 (27%) | 1,260 (63%) | 1,870 (78%) | 755 (71%) | <0.001 |
| Age (years) | 69.7 (60.2–76.9) | 66.3 (57.2–74.9) | 62.8 (55.5–71.0) | 61.2 (55.0–68.5) | <0.001 |
| Body mass index | 17.7 (17.2–18.1) | 23.3 (21.9–24.3) | 27.1 (26.0–28.3) | 32.3 (30.9–34.5) | <0.001 |
| Charlson Co-Morbidity Index score | 0.001 | ||||
| 0 | 13 (22%) | 563 (28%) | 762 (32%) | 325 (31%) | |
| 1–2 | 42 (70%) | 1,352 (68%) | 1,543 (64%) | 674 (63%) | |
| ≥3 | 5 (8%) | 76 (3.8%) | 94 (3.9%) | 66 (6%) | |
| Smoker | |||||
| Never | 13 (22%) | 445 (22%) | 487 (20%) | 238 (22%) | 0.34 |
| Stopped | 16 (27%) | 674 (34%) | 950 (40%) | 432 (41%) | <0.001 |
| Active | 31 (52%) | 868 (44%) | 956 (40%) | 393 (37%) | <0.001 |
| Diabetes mellitus | 4 (7%) | 122 (6%) | 214 (9%) | 213 (20%) | <0.001 |
| Hypertension | 33 (55%) | 1,155 (58%) | 1,437 (60%) | 708 (67%) | <0.001 |
| Medical treatment | |||||
| Statins | 44 (73%) | 1,672 (84%) | 2,034 (85%) | 916 (86%) | 0.04 |
| β Blockers | 37 (62%) | 1,527 (77%) | 1,885 (79%) | 835 (78%) | 0.010 |
| Angiotensin-converting enzyme inhibitors | 37 (62%) | 1,068 (54%) | 1,341 (56%) | 650 (61%) | 0.001 |
| Angiotensin II receptor blockers | 2 (3.3%) | 128 (6%) | 192 (8%) | 106 (10%) | 0.003 |
| Digoxin | 2 (3.3%) | 68 (3.4%) | 83 (3.5%) | 33 (3.1%) | 0.96 |
| Aspirin | 38 (63%) | 1,314 (66%) | 1,587 (66%) | 729 (69%) | 0.49 |
| Warfarin | 3 (5%) | 82 (4.1%) | 104 (4.3%) | 38 (3.6%) | 0.74 |
| Variable | BMI (kg/m 2 ) | p Value | |||
|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30.0 | ||
| (n = 60) | (n = 1,991) | (n = 2,399) | (n = 1,065) | ||
| Drug-eluting stent at index percutaneous coronary intervention (%) | 37 (61.7%) | 1,144 (57.5%) | 1,320 (55.0%) | 616 (57.8%) | 0.23 |
| Urgency of index percutaneous coronary intervention | |||||
| Acute (ST-segment myocardial infarction) | 27 (45%) | 647 (33%) | 687 (29%) | 277 (26%) | <0.001 |
| Subacute (non–ST-segment myocardial infarction or unstable angina pectoris) | 11 (18%) | 679 (34%) | 846 (35%) | 409 (38%) | 0.004 |
| Elective (stable angina pectoris) | 17 (28%) | 489 (25%) | 674 (28%) | 308 (29%) | 0.02 |
| Number of narrowed coronary vessels | 0.55 | ||||
| 1 | 35 (71%) | 974 (64%) | 1,145 (65%) | 487 (65%) | |
| 2 | 8 (16%) | 350 (23%) | 416 (24%) | 176 (24%) | |
| 3 | 6 (12%) | 196 (13%) | 191 (11%) | 82 (11%) | |
| Number of stents | 0.82 | ||||
| 1 | 54 (90%) | 1,767 (89%) | 2,104 (88%) | 934 (87.7%) | |
| 2 | 6 (10%) | 202 (10%) | 270 (11%) | 115 (10.8%) | |
| 3 | 0 (0.0%) | 21 (1.1%) | 25 (1.0%) | 15 (1.4%) | |
| 4 | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | |
| Coronary artery stented | |||||
| Left main | 1 (1.7%) | 6 (0.3%) | 17 (0.7%) | 5 (0.5%) | 0.17 |
| Left anterior descending | 33 (55%) | 941 (47%) | 1,146 (48%) | 493 (46%) | 0.56 |
| Left circumflex | 2 (3.3%) | 262 (13%) | 319 (13%) | 169 (16%) | 0.01 |
| Right | 23 (38%) | 736 (37%) | 846 (35%) | 365 (34%) | 0.44 |
| Stent diameter (mm) | 3.0 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.5 | 3.1 ± 0.5 | <0.001 |
| Stent length (mm) | 17.7 ± 7.1 | 16.2 ± 5.9 | 16.3 ± 6.0 | 16.2 ± 6.0 | 0.40 |
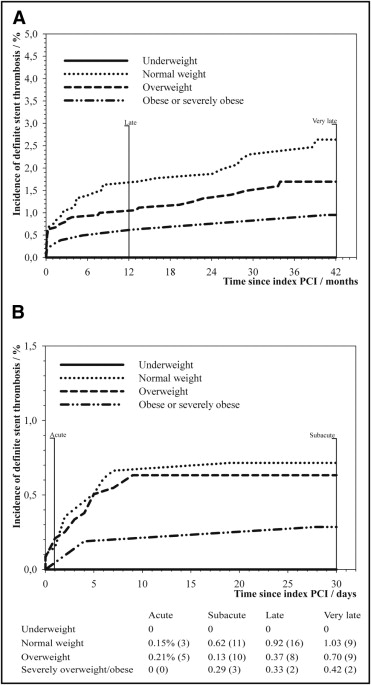
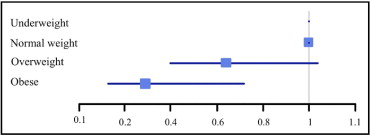
Definite stent thrombosis occurred in 78 patients at a median of 68 days (interquartile range 4 to 370) after the index PCI. Kaplan–Meier curves illustrating cumulative incidences of definite stent thrombosis are shown in Figure 1 , and hazard ratios (HRs) of definite stent thrombosis compared to normal-weight patients are illustrated in Figure 2 . Results of multivariate analyses of risk of definite stent thrombosis are presented in Table 3 . Clopidogrel did not predict definite stent thrombosis in unadjusted analysis (HR 1.17, confidence interval [CI] 0.75 to 1.83). We found that our model was very good at rank ordering patients with definite stent thrombosis from patients without with a Harrell c-statistic of 0.81 (0.77 to 0.86). We found no significant interaction between type of index stent and BMI as a continuous variable (p = 0.48) or group-wise (p = 0.29) with regard to risk of definite stent thrombosis. In addition, no interaction was found between BMI and diabetes (p = 0.83), treatment with statins (p = 0.10), PCI urgency (p = 0.12), or treatment with clopidogrel (p = 0.41). Results were not significantly changed by calculation of risk of definite stent thrombosis in number of events per 1,000 person-years (not shown). Because DESs were not used before 2003, we performed a sensitivity analysis exploring the impact of BMI on risk of definite stent thrombosis for PCIs done after 2003, which did not change the results significantly (HR 0.91, CI 0.84 to 0.98).