It is well known that chronic kidney disease is a risk factor for atherosclerosis. The present study was conducted to identify any relation between the estimated glomerular filtration rate (eGFR) and coronary plaque characteristics using integrated backscatter intravascular ultrasound (IB–IVUS), which can detect coronary plaque composition. We performed IB–IVUS for 201 consecutive patients undergoing percutaneous coronary intervention, and they were divided into 3 groups according to the eGFR values (group 1 [n = 20], ≥90 ml/min/1.73 m 2 ; group 2 [n = 123], 60 to 90 ml/min/1.73 m 2 ; and group 3 [n = 58], <60 ml/min/1.73 m 2 ). Coronary plaques in nonculprit lesions on 3-dimensional analysis were evaluated using IB–IVUS. The baseline characteristics were similar, except for older age and a greater prevalence of men in group 3. IB–IVUS showed a percentage of lipid volume of 44.7 ± 5.0% in group 1, 53.6 ± 6.2% in group 2, and 63.5 ± 6.2% in group 3 (p <0.01), with a corresponding percentage of fibrous volume of 53.9 ± 4.9%, 45.1 ± 6.0%, and 35.3 ± 6.1%, respectively (p <0.01). The eGFR correlated significantly with both parameters (r = −0.68, p <0.001 and r = 0.68, p <0.001, respectively). In conclusion, lower eGFR levels were associated with greater lipid and lower fibrous contents, contributing to coronary plaque vulnerability.
Chronic kidney disease (CKD) is well-known to be associated with adverse cardiac events. Even mild renal dysfunction should be considered an independent cardiovascular risk factor. CKD also increases the likelihood of acute coronary syndrome (ACS). It has been reported that culprit lesions in coronary arteries demonstrate angiographically mild to moderate stenosis, with disruption and erosion of vulnerable plaques the most frequent cause of ACS. Integrated-backscatter intravascular ultrasound (IB–IVUS) provides quantitative information on coronary plaque composition. Because coronary plaques containing more lipid and less fibrous tissue are predictive of future cardiac events, it is clinically important that they be detected and treated. However, few studies have assessed the relation between renal dysfunction and coronary plaque composition. The aim of the present study was therefore to analyze the coronary tissue components in patients with renal impairment using the estimated glomerular filtration rate (eGFR).
Methods
The present study was prospectively performed from January 2009 to December 2009. We evaluated the plaque components of nontarget coronary lesions with mild to moderate stenosis defined as a percentage of diameter stenosis of <50% in patients with ACS or stable angina pectoris who underwent percutaneous coronary intervention using IB–IVUS. The data from patients with poor IVUS images were excluded. In the case of stable angina pectoris, dual antiplatelet therapy (aspirin, 81 to 162 mg/day, and clopidogrel, 75 mg/day) and a statin were started ≥1 month before percutaneous coronary intervention after checking the tolerance of those drugs, if those drugs had not been previously administered.
The patients were divided into 3 groups according to the eGFR: group 1, eGFR ≥90 ml/min/1.73 m 2 ; group 2, eGFR ≥60 ml/min/1.73 m 2 but <90 ml/min/1.73 m 2 ; and group 3, eGFR <60 ml/min/1.73 m 2 . The calculations were made using the Modification of Diet in Renal Disease study equation modified with the Japanese coefficient: eGFR (ml/min/1.73 m 2 ) = 194 × serum creatinine −1.094 × age −0.287 × 0.739 (for women). Diabetes mellitus was defined as medication dependent, including oral antihyperglycemic drugs and insulin, or previously known diabetes, a fasting plasma glucose concentration >126 mg/dl, or a randomized plasma glucose concentration >200 mg/dl. Dyslipidemia was defined as medication-dependent or previously known dyslipidemia, low-density lipoprotein cholesterol of ≥140 mg/dl, or total cholesterol of ≥220 mg/dl. Hypertension was defined as medication-dependent, previously known or systolic blood pressure ≥140 mm Hg, and/or diastolic blood pressure ≥90 mm Hg. Smoking was defined as positive if patients were smoking currently or had stopped smoking within 6 months before the study. ACS included acute myocardial infarction and unstable angina pectoris. Acute myocardial infarction was diagnosed by the elevation of ≥1 positive biomarker (creatine kinase, creatine kinase-MB, or troponin), distinctive electrocardiographic findings, and continuous acute chest pain. Unstable angina pectoris was defined as angina with a gradual increase in chest pain or angina in a state of rest.
Quantitative coronary angiography was performed using the CAG analysis system (CAAS, Pie Medical, Maastricht, The Netherlands). The end-diastolic phase was selected for quantitative coronary angiographic analysis. Using the outer diameter of the contrast-filled guiding catheter as the reference standard, the minimum lumen diameter, reference lumen diameter, and percentage of diameter stenosis was measured from the orthogonal projections.
Nontarget lesions were defined as vessels with <50% stenosis of the lumen diameter on coronary angiography, in addition to the percentage of plaque area >20% at the lumen cross-sectional area and >10 mm distant from the target lesions. Qualitative and quantitative analyses of conventional IVUS were assessed according to the criteria of the American College of Cardiology Clinical Expert Consensus document on IVUS. Conventional IVUS images were acquired using a commercially available IVUS imaging system (Galaxy, Boston Scientific, Natick, Massachusetts) using a 40-MHz mechanically rotating IVUS catheter with motorized catheter pullback (0.5 mm/s). The IVUS catheter was advanced >10 mm beyond the nontarget lesion, and automated pullback was performed to a point proximal to the nontarget lesion. A total of 5 images were assessed at an axial interval of 1 mm for each plaque.
Conventional 2-dimensional IVUS image analysis was conducted for the lumen cross-sectional area, external elastic membrane, cross-sectional area (CSA), and plaque plus media CSA (plaque plus media CSA = external elastic membrane CSA − lumen cross-sectional area) using the software of the IVUS system. Conventional 3-dimensional image analysis was used to compute the vessel volume, lumen volume, and total plaque volume (sum of external elastic membrane, lumen CSA, and plaque plus media CSA at an axial interval of 1 mm for the analyzed segments).
A personal computer installed with custom software (IB–IVUS, YD Company, Ltd., Nara, Japan) was connected to the conventional IVUS system to obtain the radiofrequency signal trigger output. Ultrasound backscattered signals were obtained with a 40-MHz mechanically rotating IVUS catheter at a speed of 0.5 mm/s. The integrated backscatter values for each tissue component were calculated as an average power using a fast Fourier transform, measured in decibels. The fibrous area, lipid area, and high signal area (a part of the calcification on the inner surface) were determined by the definition of the integrated backscatter values for each of the 3 histologic categories. The percentage of fibrous area (fibrous area divided by plaque area) and the percentage of lipid area (lipid area divided by plaque area) were calculated by the IB–IVUS system automatically. The percentage of high signal area (high signal area divided by plaque area) and percentage of fibrous volume (fibrous volume divided by plaque volume), lipid volume (lipid volume divided by plaque volume), and high signal volume (high signal volume divided by plaque volume) were also calculated. The 3-dimensional IVUS analysis of lipid volume, fibrous volume, and high signal volume was performed with the sums of the fibrous, lipid, and high signal areas in each CSA at 1-mm axial intervals for the analyzed segments. Representative cases are shown in Figure 1 .
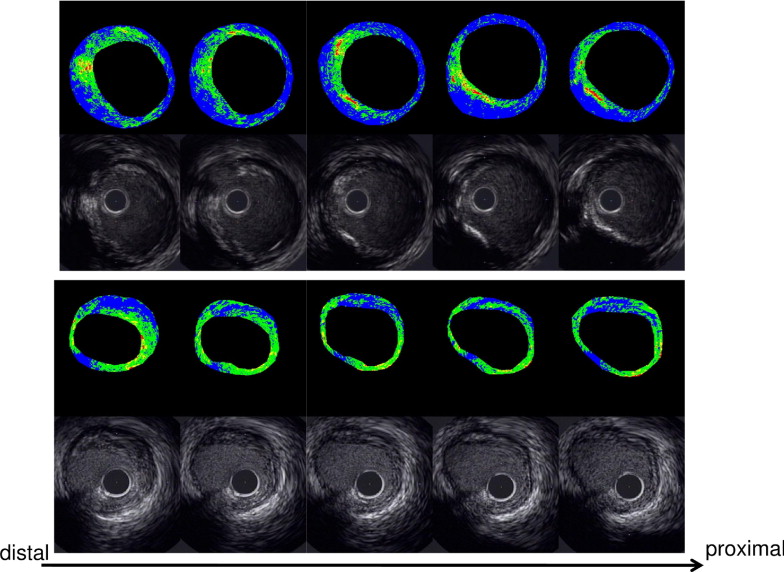
Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS, Chicago, Illinois). Continuous variables are presented as the mean ± SD. Various parameters were compared among the 3 groups using analysis of variance or the chi-square test. Simple linear regression analysis was applied to study the relations between the eGFR and IVUS parameters, and multivariate regression analysis was done adjusting for predefined variables. A p value of <0.05 indicated statistical significance.
Results
A total of 201 patients who had nontarget lesions without significant stenosis were evaluated in the present study. Of those enrolled, 20 had an eGFR of ≥90 ml/min/1.73 m 2 (group 1), 123 had an eGFR of ≥60 ml/min/1.73 m 2 but <90 ml/min/1.73 m 2 (group 2), and 58 had an eGFR <60 ml/min/1.73 m 2 (group 3).
The coefficients between the measurements of the percentage of lipid volume and percentage of fibrous volume taken by 2 physicians who assessed the IVUS measurements independently were well correlated (r = 0.92, p <0.01, and r = 0.93, p <0.01).
The baseline clinical characteristics are listed in Table 1 . Patients with a lower eGFR were more likely to be older and male. However, no significant differences were found in the frequency of hypertension, diabetes mellitus, dyslipidemia, or smoking status among the 3 groups.
| Characteristic | All (n = 201) | eGFR (ml/min/1.73 m 2 ) | p Value | ||
|---|---|---|---|---|---|
| ≥90 (n = 20) | ≥60 but <90 (n = 123) | <60 (n = 58) | |||
| Age (years) | 67.0 ± 9.5 | 60.3 ± 7.6 | 67.4 ± 9.5 ⁎ | 70.8 ± 8.8 † | <0.01 |
| Men | 150 (75%) | 10 (50%) | 91 (74%) | 49 (84%) | <0.01 |
| Body mass index (kg/m 2 ) | 24.1 ± 3.5 | 24.7 ± 2.8 | 24.0 ± 3.8 | 24.1 ± 3.1 | 0.7 |
| Hypertension ‡ | 131 (65%) | 10 (50%) | 79 (64%) | 42 (72%) | 0.18 |
| Diabetes | 89 (44%) | 8 (40%) | 52 (42%) | 29 (50%) | 0.57 |
| Dyslipidemia § | 152 (76%) | 14 (70%) | 95 (77%) | 43 (74%) | 0.74 |
| Smoker | 68 (34%) | 9 (45%) | 38 (31%) | 21 (36%) | 0.42 |
| Acute coronary syndrome | 26 (13%) | 3 (15%) | 18 (15%) | 5 (9%) | 0.51 |
| Hemoglobin A1c (%) | 6.5 ± 1.4 | 6.4 ± 1.5 | 6.5 ± 1.4 | 6.6 ± 1.5 | 0.46 |
| Total cholesterol (mg/dl) | 193 ± 40 | 197 ± 44 | 195 ± 37 | 188 ± 44 | 0.46 |
| Low-density lipoprotein cholesterol (mg/dl) | 109 ± 37 | 116 ± 37 | 110 ± 36 | 104 ± 39 | 0.42 |
| High-density lipoprotein cholesterol (mg/dl) | 51 ± 14 | 48 ± 16 | 52 ± 15 | 49 ± 12 | 0.5 |
| Triglycerides (mg/dl) | 170 ± 110 | 161 ± 73 | 170 ± 103 | 173 ± 135 | 0.92 |
| Creatinine (mg/dl) | 0.86 ± 0.28 | 0.56 ± 0.08 | 0.77 ± 0.12 ⁎ | 1.17 ± 0.31 ∥ | <0.01 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 68.9 ± 17.5 | 99.7 ± 9.3 | 73.6 ± 8.2 ⁎ | 48.3 ± 9.2 ∥ | <0.01 |
| Previous myocardial infarction | 63 (31%) | 9 (45%) | 34 (28%) | 20 (34%) | 0.25 |
| Previous percutaneous coronary intervention | 85 (42%) | 6 (30%) | 50 (41%) | 29 (50%) | 0.25 |
| Previous coronary artery bypass grafting | 6 (3%) | 1 (5%) | 3 (2%) | 2 (3%) | 0.80 |
| Analyzed coronary artery | 0.25 | ||||
| Right | 63 (31%) | 9 (45%) | 34 (28%) | 20 (34%) | |
| Left anterior descending | 113 (56%) | 7 (35%) | 73 (59%) | 33 (57%) | |
| Left circumflex | 25 (12%) | 4 (20%) | 16 (13%) | 5 (9%) | |
| Medication | |||||
| Aspirin | 186 (93%) | 17 (85%) | 114 (93%) | 55 (95%) | 0.35 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 109 (54%) | 8 (40%) | 64 (52%) | 37 (64%) | 0.13 |
| Calcium channel blocker | 90 (45%) | 6 (30%) | 56 (46%) | 28 (48%) | 0.35 |
| β Blocker | 32 (16%) | 1 (5%) | 20 (16%) | 11 (19%) | 0.33 |
| Pioglitazon | 14 (7%) | 1 (5%) | 8 (7%) | 5 (9%) | 0.82 |
| Insulin | 19 (9%) | 1 (5%) | 12 (10%) | 6 (10%) | 0.77 |
⁎ p <0.01 compared to group 1;
† p <0.05 compared to group 2 and p <0.01 compared to group 1;
∥ p <0.01 compared to groups 1 and 2.
‡ Defined as medication-dependent, previously known, or systolic blood pressure ≥140 mm Hg, and/or diastolic blood pressure ≥90 mm Hg.
§ Defined as medication-dependent or previously known dyslipidemia, low-density lipoprotein cholesterol ≥140 mg/dl, or total cholesterol ≥220 mg/dl.
Quantitative coronary angiographic data and the gray-scale and IB–IVUS findings are summarized in Table 2 . No significant differences were seen in the minimum lumen diameter, percentage of diameter stenosis, or reference diameter among the 3 groups. In the 2-dimensional gray-scale IVUS analysis, the percentage of plaque area, plaque area, and vessel area at the minimum luminal CSA were significantly larger in group 3 than in groups 1 and 2 ( Table 2 ).
| Characteristic | All | eGFR | p Value | ||
|---|---|---|---|---|---|
| ≥90 | ≥60 but <90 | <60 | |||
| Quantitative coronary angiography | |||||
| Reference vessel diameter (mm) | 3.21 ± 0.78 | 3.14 ± 0.61 | 3.15 ± 0.72 | 3.34 ± 0.94 | 0.29 |
| Minimum lumen diameter (mm) | 2.69 ± 0.74 | 2.63 ± 0.48 | 2.65 ± 0.72 | 2.79 ± 0.87 | 0.47 |
| Diameter stenosis (%) | 16.6 ± 8.3 | 16.1 ± 6.1 | 16.6 ± 8.6 | 16.7 ± 8.6 | 0.96 |
| 2-Dimensional intravascular ultrasound (gray scale) | |||||
| Percentage of plaque area | 46.5 ± 11.9 | 43.4 ± 11.3 | 45.2 ± 11.6 | 50.2 ± 12.0 ⁎ | 0.014 |
| Plaque area (mm 2 ) | 8.1 ± 3.1 | 6.7 ± 3.1 | 7.5 ± 2.6 | 9.8 ± 3.2 † | <0.01 |
| Lumen area (mm 2 ) | 9.2 ± 3.2 | 8.6 ± 3.2 | 9.0 ± 3.0 | 9.7 ± 3.5 | 0.28 |
| Vessel area (mm 2 ) | 17.3 ± 4.6 | 15.3 ± 5.1 | 16.6 ± 3.8 | 19.5 ± 5.1 † | <0.01 |
| 2-Dimensional intravascular ultrasound (integrated backscatter–intravascular ultrasound) | |||||
| Percentage of lipid area | 55.4 ± 9.5 | 45.1 ± 7.8 | 53.0 ± 7.5 ‡ | 64.0 ± 7.1 † | <0.01 |
| Percentage of fibrous area | 43.2 ± 9.4 | 53.4 ± 7.1 | 45.6 ± 7.2 ‡ | 34.4 ± 7.7 † | <0.01 |
| Percentage of calcified area | 1.2 ± 1.3 | 1.3 ± 1.2 | 1.3 ± 1.4 | 1.0 ± 1.2 | 0.21 |
| 3-Dimensional intravascular ultrasound (gray scale) | |||||
| Percentage of plaque volume | 46.4 ± 11.5 | 41.5 ± 12.3 | 45.2 ± 11.1 | 50.6 ± 11.0 † | <0.01 |
| Plaque volume (mm 3 ) | 40.4 ± 15.0 | 33.6 ± 15.3 | 37.2 ± 12.6 | 49.5 ± 15.8 † | <0.01 |
| Lumen volume (mm 3 ) | 46.0 ± 15.6 | 44.3 ± 15.9 | 45.1 ± 14.9 | 48.6 ± 17.1 | 0.33 |
| 3-Dimensional intravascular ultrasound (integrated backscatter–intravascular ultrasound) | |||||
| Vessel volume (mm 3 ) | 87.1 ± 22.9 | 83.0 ± 31.6 | 82.6 ± 18.3 | 97.9 ± 24.9 † | <0.01 |
| Percentage of lipid volume | 55.6 ± 8.4 | 44.7 ± 5.0 | 53.6 ± 6.2 ‡ | 63.5 ± 6.2 † | <0.01 |
| Percentage of fibrous volume | 43.1 ± 8.2 | 53.9 ± 4.9 | 45.1 ± 6.0 ‡ | 35.3 ± 6.1 † | <0.01 |
| Percentage of calcified volume | 1.3 ± 1.1 | 1.4 ± 1.2 | 1.3 ± 1.1 | 1.2 ± 1.2 | 0.61 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


