Hospital-acquired anemia (HAA) is common, often develops in the absence of bleeding, and is associated with poor outcomes in patients with acute myocardial infarction (AMI). It is unknown whether red cell distribution width (RDW) and mean corpuscular volume (MCV), which are routinely available markers of iron deficiency, are associated with development of HAA during AMI. We studied 15,133 patients with AMI without anemia at admission. HAA was defined by nadir hemoglobin levels below age-, gender-, and race-specific thresholds and moderate–severe HAA was defined as nadir hemoglobin ≤11 g/dl. We examined the association between low MCV (<80 fL) and/or increased RDW (>15%) on patients’ initial complete blood cell count and moderate–severe HAA using multivariable modified Poisson regression. Moderate–severe HAA was more common in patients with high RDW and low MCV (45.5%), high RDW and MCV ≥80 fL (33.0%), and normal RDW and low MCV (28.0%) than in those with normal RDW and MCV (18.3%, p <0.001). Compared to patients with normal RDW and MCV, those with increased RDW and low MCV (relative risk 1.72, 95% confidence interval 1.57 to 1.87), increased RDW and MCV ≥80 fL (relative risk 1.28, 95% confidence interval 1.16 to 1.42), or normal RDW and low MCV (relative risk 1.34, 95% confidence interval 1.08 to 1.65) were independently more likely to develop moderate–severe HAA. In conclusion, increased RDW and low MCV were independent predictors of moderate–severe HAA.
Development of hospital-acquired anemia (HAA) in patients with normal hemoglobin at the time of admission with acute myocardial infarction (AMI) is common and is associated with worse health status, higher mortality, and persistent anemia after hospital discharge. In contrast to chronic anemia, HAA may be preventable if modifiable risk factors for HAA are identified and treated. One potential opportunity to manage HAA may be recognizing and treating iron deficiency. Iron deficiency is common in other cardiac conditions including heart failure, and iron supplementation has been shown to improve health status in these patients. However, routine screening of all patients with AMI would be costly and justifiable only if its identification were associated with adverse outcomes such as HAA. Although the formal diagnosis of iron deficiency would require the use of nonroutine tests, red blood cell indices including red cell distribution width (RDW) and mean corpuscular volume (MCV) are available from complete blood cell counts that are universally obtained at time of AMI admission. If these red blood cell indices were associated with HAA, they could be used to prospectively identify patients who may benefit from further evaluation of iron stores and iron replacement and other HAA prevention and management strategies including bleeding prevention and judicious use of phlebotomy. Accordingly, we examined the relation of RDW and MCV to new-onset HAA in consecutive patients with AMI hospitalized at 56 hospitals in the Health Facts database.
Methods
We used the Health Facts database to study the prevalence of abnormal red blood cell indices and the association between abnormal RDW or MCV and HAA in patients with AMI. Health Facts contains de-identified data from the Cerner electronic medical record for patients admitted to participating hospitals from January 1, 2000 through December 31, 2008. The database includes hospital characteristics, patients’ demographics, medical history, and co-morbidities, laboratory studies, medications, procedures, and complications.
We defined anemia using age-, gender-, and race-specific criteria described by Beutler and Waalen (hemoglobin <13.7 g/dl for white men 20 to 59 years old, <13.2 g/dl for white men ≥60 years old, <12.9 g/dl for black men 20 to 59 years old, <12.7 g/dl for black men ≥60 years old, <12.2 g/dl for white women, <11.5 g/dl for black women). This classification was derived from contemporary cohorts and identifies anemia more accurately than the World Health Organization definition. In the absence of race-specific data for patients of other racial backgrounds (<5% of patients in Health Facts), we applied diagnostic criteria for Caucasians. Patients were classified as having HAA if their initial hemoglobin was above the diagnostic thresholds but their lowest (nadir) hemoglobin was below the thresholds for anemia. HAA was classified as mild if the nadir hemoglobin was >11.0 g/dl and moderate–severe if the nadir hemoglobin was ≤11.0 g/dl. Because moderate–severe HAA, but not mild HAA, has been shown to be prognostically important, we selected moderate–severe HAA as the outcome for the present study.
RDW measures anisocytosis and higher values indicate greater variability in the size of circulating erythrocytes. An increased RDW was defined as a value >15%. Microcytosis was defined as an MCV <80 fL. In previous studies of anemic patients, increased RDW with low or normal MCV has been shown to be associated with iron deficiency, although low MCV can also be seen in other conditions such as anemia of chronic disease and thalassemia and a high RDW can been seen in the setting of folate or vitamin B12 deficiency, hemolysis, liver disease, or bone marrow infiltration. Studying these 2 markers is important because increased RDW may precede the development of low MCV in iron-deficient patients who have not yet developed anemia. Because the use of these metrics may support identification and interventions in patients at risk for HAA during hospitalization, we used each patient’s first RDW and MCV as the exposure variable of interest. Screening for iron deficiency was defined as any laboratory result for ferritin. Because patients with chronic kidney disease and heart failure are considered iron deficient at higher thresholds than patients without these conditions, we defined iron deficiency as a ferritin level <100 ng/ml or 100 to 299 ng/ml when transferrin saturation was <20%, consistent with guidelines for diagnosing iron deficiency in patients with chronic renal disease and heart failure. In the absence of these conditions, ferritin <45 ng/ml was used to identify iron deficiency.
We included all patients with a primary discharge diagnosis of AMI as defined by International Classification of Diseases, Ninth Revision, Clinical Modification codes 410.xx. We further confirmed AMI by requiring ≥1 increased cardiac biomarker (troponin or creatine kinase-MB) and excluding patients discharged alive within the first 24 hours of hospitalization. Full inclusion and exclusion criteria are presented in Figure 1 . We excluded patients transferred from other hospitals (laboratory data may not be available) or from hospice (goals of care differ). To improve generalizability, we then excluded patients from hospitals treating <20 patients with AMI during the study period and patients with lengths of stay >31 days. We also excluded patients who underwent coronary bypass grafting during index admission because the causes and outcomes of HAA are different in these patients. Patients who did not have hemoglobin assessed within the first 24 hours of admission had <2 hemoglobin values and patients anemic at admission were excluded. Of these 17,676 patients without anemia on admission from 57 hospitals, 2,452 patients were contributed by a single hospital that did not report any RDW data. Accordingly, this site was excluded. Missing data for MCV (n = 39) and RDW (n = 52) at other hospitals were minimal. After excluding these patients, we analyzed the 15,133 patients with known values for RDW and MCV.
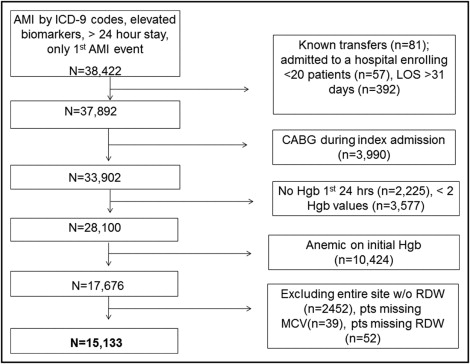
We compared patient characteristics, laboratory values, in-hospital treatments, and complications of patients who developed moderate–severe HAA to those who did not. We presented categorical data as frequencies and compared differences between groups using chi-square tests. Continuous variables were reported as mean ± SD or median (interquartile range) and differences between groups were compared using Student’s t tests or Wilcoxon rank-sum tests, as appropriate. To identify the independent association between red blood cell indices and moderate–severe HAA, we fit hierarchical multivariable modified Poisson regression models. Because RDW and MCV provide complementary diagnostic information, we modeled the primary exposure variable by defining groups based on the 2 markers. Patients with an increased RDW and MCV <80 fL, an increased RDW with MCV ≥80 fL, or a normal RDW with MCV <80 fL were compared to a reference group with a normal RDW and MCV ≥80 fL. We adjusted for age, gender, race (Caucasian vs other), co-morbidities (chronic kidney disease, heart failure, hypertension, diabetes, and previous MI), AMI type (ST-segment elevation vs non–ST-segment elevation), complications (acute renal failure, cardiogenic shock, and mechanical ventilation), and treatments (cardiac catheterization or angioplasty and use of thrombolytics, intravenous heparin, bivalirudin, thienopyridines, aspirin, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, or β blockers).
We conducted sensitivity analyses to assess the robustness of our findings. Because the prevalence of underlying conditions that might influence RDW or MCV such as thalassemia or sickle cell anemia differ across racial backgrounds, we repeated our primary analyses after stratifying by race (Caucasian vs other). Because abnormal red blood cell indices might reflect the prognostic impact of lower hemoglobin, we added patients’ initial hemoglobin to the multivariable model and reassessed the relation of RDW and MCV to moderate–severe HAA. In addition, we conducted exploratory analyses to examine the relation between red cell indices and iron deficiency in the subgroup of patients who had assessment of iron stores during hospitalization with AMI. We identified the frequency of iron deficiency in each category of RDW and MCV in patients with ferritin assessment.
Results
Moderate–severe HAA was common (n = 3,088, 20.4%). There were important differences between patients who did and did not develop moderate–severe HAA ( Table 1 ). Few patients had assessment of iron stores (n = 467, 3.1%), although iron testing was more common in patients with moderate–severe HAA than in those without (319, 10.3%, vs 148, 1.2%, p <0.001). Mean RDW was higher (14.2 ± 2.5% vs 13.6 ± 2.3%, p <0.001) and a larger proportion of patients had an MCV <80 fL (122, 4.0%, vs 194, 1.6%, p <0.001) in those with moderate–severe HAA. Most patients (n = 13,100, 86.6%) had normal RDW and MCV, whereas 1,717 (11.4%) had an increased RDW but normal MCV, 125 (0.8%) had a normal RDW but low MCV, and 191 (1.3%) had an increased RDW and low MCV.
| Variable | Moderate–Severe HAA | p Value | |
|---|---|---|---|
| Yes (n = 3,088) | No (n = 12,045) | ||
| Age (years), mean ± SD | 71.8 ± 12.9 | 64.7 ± 14.5 | <0.001 |
| Caucasian | 2,499 (80.9%) | 10,357 (86.0%) | <0.001 |
| Women | 2,168 (70.2%) | 4,073 (33.8%) | <0.001 |
| Heart failure | 1,233 (39.9%) | 2,705 (22.5%) | <0.001 |
| Hypertension | 1,549 (50.2%) | 6,743 (56.0%) | <0.001 |
| Previous percutaneous coronary intervention | 100 (3.2%) | 819 (6.8%) | <0.001 |
| Chronic kidney disease | 422 (13.7%) | 576 (4.8%) | <0.001 |
| Peripheral arterial disease | 92 (3.0%) | 253 (2.1%) | 0.004 |
| Diabetes mellitus | 1,009 (32.7%) | 3,118 (25.9%) | <0.001 |
| Previous coronary artery bypass grafting | 93 (3.0%) | 597 (5.0%) | <0.001 |
| Previous myocardial infarction | 122 (4.0%) | 813 (6.7%) | <0.001 |
| Current smoker | 426 (13.8%) | 3,928 (32.6%) | <0.001 |
| History of stroke or transient ischemic attack | 151 (4.9%) | 288 (2.4%) | <0.001 |
| ST-segment elevation myocardial infarction | 1,314 (42.6%) | 5,115 (42.5%) | 0.931 |
| Acute renal failure | 450 (14.6%) | 433 (3.6%) | <0.001 |
| Cardiogenic shock | 301 (9.7%) | 282 (2.3%) | <0.001 |
| Mechanical ventilation during hospitalization | 353 (11.4%) | 369 (3.1%) | <0.001 |
| Bleeding event | 419 (13.6%) | 368 (3.1%) | <0.001 |
| Bleeding event type | <0.001 | ||
| Gastrointestinal site | 172 (41.1%) | 93 (25.3%) | |
| Intracranial site | 13 (3.1%) | 31 (8.4%) | |
| Other site | 234 (55.8%) | 244 (66.3%) | |
| Thienopyridine | 1,986 (64.3%) | 8,077 (67.1%) | 0.004 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 2,093 (67.8%) | 7,855 (65.2%) | 0.007 |
| Aspirin | 2,651 (85.9%) | 10,298 (85.5%) | 0.592 |
| β Blocker | 2,654 (86.0%) | 10,383 (86.2%) | 0.743 |
| Diuretics | 1,672 (54.2%) | 3,766 (31.3%) | <0.001 |
| Glycoprotein IIb/IIIa antagonist | 1,473 (47.7%) | 6,005 (49.9%) | 0.034 |
| Intravenous heparin | 1,606 (52.0%) | 5,843 (48.5%) | <0.001 |
| Bivalirudin | 108 (3.5%) | 531 (4.4%) | 0.025 |
| Fibrinolytic | 179 (5.8%) | 571 (4.7%) | 0.016 |
| Warfarin | 386 (12.5%) | 950 (7.9%) | <0.001 |
| In-hospital cardiac catheterization | 1,884 (61.0%) | 8,413 (69.8%) | <0.001 |
| In-hospital percutaneous coronary intervention | 1,400 (45.3%) | 6,415 (53.3%) | <0.001 |
| Initial hemoglobin (g/dl), mean ± SD | 13.64 ± 1.16 | 14.71 ± 1.28 | <0.001 |
| Minimum hemoglobin (g/dl), mean ± SD | 9.77 ± 1.16 | 13.10 ± 1.24 | <0.001 |
| Initial creatinine (mg/dl), median (interquartile range) | 1.10 (0.88–1.54) | 1.00 (0.88–1.20) | <0.001 |
| Initial mean corpuscular volume (fL), mean ± SD | 90.1 ± 5.8 | 90.3 ± 4.8 | 0.064 |
| Mean corpuscular volume <80 fL | 122 (4.0%) | 194 (1.6%) | <0.001 |
| Mean corpuscular volume >100 fL | 112 (3.6%) | 274 (2.3%) | <0.001 |
| Initial red cell distribution width (%), mean ± SD | 14.2 ± 2.5 | 13.6 ± 2.3 | <0.001 |
| Red cell distribution width >15% | 653 (21.1%) | 1,255 (10.4%) | <0.001 |
| Ferritin assessed | 319 (10.3%) | 148 (1.2%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


