Reconstruction of the Vertebral Artery
INDICATIONS FOR SURGERY
Reconstruction of the VA is indicated in patients with VBI symptoms and appropriate VA lesions. Other than in reconstructions done for extracranial VA aneurysms, I have operated only on two patients with asymptomatic VA lesions: at the beginning of my experience I did a proximal VA transposition in a young woman with severe post-radiation (95%) bilateral VA stenosis and absent posterior communicantes. The other patient had bilateral ICA and VA occlusions and I did a revascularization of a distal VA in preparation for cardiopulmonary bypass. All other VA reconstructions have been done in symptomatic patients in whom the VA lesion was shown, or could be presumed, to be the cause of the neurologic symptoms.
Patients present with either low-flow or embolic symptoms.i Low-flow symptoms are iterative, caused by the same neck movement (e.g., rotation to one side or extension) and can be reproduced on clinical examination. They do not result in brain infarction. The potential morbidity for these patients is not a stroke but rather the trauma that may follow a bout of vertigo or syncope at an inopportune time (driving, climbing a ladder).
Embolic symptoms, on the contrary, have varied topography depending on where the microembolus impacts and are not triggered by any specific motion. The MRI will show a cerebellar or brain stem infarction. They also have a particularly ominous prognosis: posterior brain infarction is three times more deadly than anterior infarction.
In patients presenting with low-flow symptoms secondary to severe VA stenosis, the dominant VA is the one that must be reconstructed. In determining what constitutes a critical reduction of diameter, I have followed the heuristic that 75% of the total inflow needs to be compromised to justify ascribing the symptoms to low flow. This means that a patient with an equivalent VA should have bilateral >75% stenosis in both arteries or, if there is clearly a dominant or single VA, the latter should have >75% of its diameter compromised.
Patients with low-flow symptoms, in addition to their significant VA lesions, have minute or absent posterior communicating arteries. This lack of a compensatory mechanism from the carotid territory potentiates the inflow deficit imposed by the stenotic vertebral lesion. Patients with low-flow VBI secondary to rotation-extension of the neck need to have the cause-effect relationship proven by dynamic
arteriography (see Section “Dynamic Compression of the Vertebral Artery (Bow Hunter Syndrome)” in Chapter 3).
arteriography (see Section “Dynamic Compression of the Vertebral Artery (Bow Hunter Syndrome)” in Chapter 3).
In patients with embolic VBI, the VA responsible for the embolization must be identified (see Section “Divergence vs. Convergence” in Chapter 2) in order to plan treatment. In some cases with bilateral VA disease, the responsible VA can be deduced from the location of the cerebellar infarction. However, when the infarction is in the inferior cerebellar, basilar, or posterior cerebral artery territory and both VAs are patent and diseased, one may not be able to presume what side is causing the symptoms.
The presentation of my experience with VA reconstruction to an audience of clinicians invariably raises the demand for a prospective randomized trial to demonstrate the validity of VA reconstruction. But the known contrasting outcomes of low-flow (physical limitations, trauma) and embolic disease (severe incapacitating stroke or death) render useless any conclusion that could be reached by such a study.
Assume that patients with symptoms of VBI and evidence of severe VA disease are randomized into operative and non-operative therapy. Assume further that we choose the traditional end-points of death and stroke and that the distribution of etiology would be similar to what we see in practice: approximately 70% having low-flow symptoms and 30% embolic symptoms.
As clinical practice dictates today, those patients with VBI induced by rotation of the head (transient low-flow) would not be entered into the study because dynamic arteriography is not part of the clinical workup today and this is the only test that demonstrates the cause-effect relationship between rotation of the head and vertebral flow arrest. Even if their workup were to involve a routine CTA, the latter would fail to show any vertebral lesion. The rest of the patients with low flow would not show any improvement in end-points with surgery because the death/stroke rate in these patients is similar to that of a normal population (their morbidity is limited to some physical disability and to trauma from loss of equilibrium at a critical moment).
Those patients with VBI secondary to embolization that were randomized to surgery would be exposed to a death/stroke risk of <2% once, but the beneficial effects of the operation in this subset will be diluted by the non-effect on death and stroke end-points noted in the larger low-flow group. And in this subset with an embolic etiology, those randomized to medical therapy will experience strokes that have a lethal outcome that is three times greater than is seen in strokes due to carotid disease.
The outcome of such an indiscriminate randomization (not differentiating low-flow from embolic etiologies) would not improve our management of VBI and would likely result in greater disability and death (from those patients with an embolic pathology).
PROXIMAL VERTEBRAL RECONSTRUCTION
Nearly all proximal VA reconstructions are done by transposing its first segment to the neighboring CCA. This technique requires only limited dissection, one anastomosis, and has excellent primary patency rates (99% at 5 years).ii
A roll under the patient’s shoulder keeps the head slightly extended and turned to the opposite side with its weight well supported by padding. The incision is S-shaped 6 to 7 cm long. The flaps of the wound are undermined three-fourth of an inch and the two bellies of the sternomastoid muscle are separated (Fig. 6.1A). The omohyoid muscle is divided. The IJV is retracted laterally with the vagus nerve. The CCA is exposed extending its dissection proximally into the mediastinum.
On the left side, the thoracic duct is identified as it emerges behind the carotid turning toward the jugulo-subclavian venous confluent. The duct is ligated and cut (Fig. 6.1B). On the right side, it is common to encounter one or two small lymph ducts that are likewise individually ligated and divided. The next landmark to look for is the vertebral vein (Fig. 6.1C) because the VA will generally be found posterior to it. The vertebral vein follows a similar course to the VA that lies posterior to it. The vein, which is slightly larger than the artery, has a straight trajectory, while the corresponding first segment of the VA can be tortuous and loop upon itself. The vein has branches; the V1 segment of the artery does not. At times, the VA is not found after dividing the vertebral vein. This situation arises with three anatomical variants: (1) when the VA exits the SA more lateral, distal to the level of origin of the internal mammary artery, (2) when the VA exits the posterior wall of the SA and runs transversely to enter, abnormally, the cervical spine at the level of C7, and (3) when the Lt VA enters the cervical spine at C5 or C4, as it does when it takes origin directly from the arch.
As the VA is dissected, the intermediate ganglion is found resting on it. The sympathetic chain that links the intermediate and the stellate ganglia crosses obliquely the VA (Fig. 6.1D). The inferior thyroid artery is divided as it crosses the field transversely entering a fenestration of the sympathetic chain. The VA is dissected from its origin to its disappearance under the lower edge of the longus colli muscle at C6. Under this musculotendinous edge, there is a vein that crosses over the VA and should be controlled with bipolar cautery. The midportion of V1 is freed from the overlying intermediate ganglion without severing the rami that exit the ganglion medially and laterally.
After adequate heparinization, the VA is occluded at the level of the longus colli with a Heifitz or a Schwartz microclip. The origin of the VA is suture ligated close to its origin and then divided placing a hemoclip on the stump for added security. Once the artery is divided, it is pulled through the sympathetic ring that surrounds it (Fig. 6.1D) at the level of the intermediate ganglion and is brought next to the CCA to verify that it will reach with ease the site planned for transposition. Damage to the intermediate ganglion or its rami will result in an incomplete Horner’s (ptosis and miosis, but no anhydrosis). The approximate site for transposition to the posterior wall of CCA is marked with a pen.
If there is a tongue of the ostial plaque still present in the distal free end of the VA, a very short, superficial, eversion endarterectomy will remove this fragment of the plaque.
To facilitate the anastomosis, the CCA is rotated to bring the posterior wall into a lateral position (Fig. 6.1E). The artery is then occluded with a baby Satinsky clamp that allows the assistant to hold the CCA next to the VA during the anastomosis. The arteriostomy in the CCA wall is made with a 5 mm aortic punch and the VA is anastomosed to it with an oblique end-to-side junction with 7-0 polypropylene. After proper forward and back bleeding flow is restored into the distal carotid and distal VA.
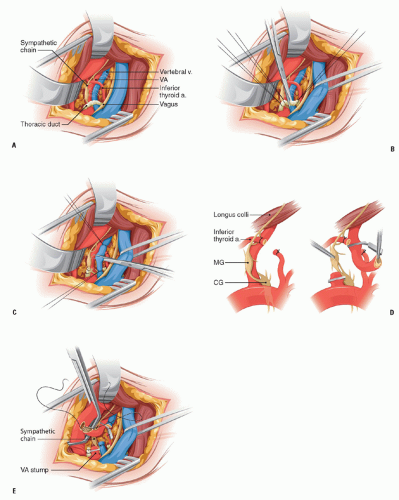 FIGURE 6.1. (A) The anatomic elements of the supraclavicular approach to V1. (B) Division of the thoracic duct as it emerges from behind the CCA to empty in the jugulo-subclavian confluent. (C) Division of the vertebral vein. (D) Extricating the VA from the middle ganglion and sympathetic chain. (E) Anastomosis of the VA to the posterior wall of the CCA. |
When the quality of the neighboring CCA is inadequate to transpose the VA to it, the reconstruction of the latter can be based on the ipsilateral SA by means of a subclavian vertebral bypass.2, 3 This alternative may be needed in those cases where the VA enters the transverse process of C7 and the length of available artery is insufficient to reach the CCA.
Reconstruction of the VA based on the SA requires exposure of the second portion of the latter. For this the incision is parallel to the clavicle. The clavicular head of the sternomastoid muscle is cut close to its insertion in the clavicle. The omohyoid muscle is divided and the prescalene fat pad is dissected along its superior, medial, and inferior edges and flipped away from the dissection field leaving lateral attachments as a hinge. The phrenic nerve, which runs on the surface of the scalenus anticus muscle, is gently dissected from the latter. Division of the scalenus anticus exposes the SA which is dissected for the length needed to find the best site for clamping it. Medially, the VA is isolated in a fashion similar to the one described above for its transposition. The distal anastomosis is done first.
The proximal VA is divided and suture ligated. If there is enough artery above the intermediate ganglion, the artery may be divided immediately above the ganglion to avoid dissecting around it. The distal end of the VA should be no less than 1.5 cm from the lower edge of the longus colli muscle to have secure control of the VA below this muscle. The artery is spatulated to construct the anastomosis to the saphenous vein graft. Once the distal anastomosis is completed, attention is turned to the SA. A baby Satinsky clamp is best suited to control the SA. The arteriostomy in the superior wall of the SA is made with a 5 mm aortic punch, and the graft is trimmed to appropriate length and is anastomosed to the SA.
DISTAL VERTEBRAL RECONSTRUCTION
The reconstruction of the distal VA can be accomplished through different techniques: the most frequent choice is a vein bypass from the CCA (Fig. 6.2). Other options (Fig. 6.3) are transposition of the ECA to the VA, transposition of the occipital artery to the VA, and transposition of the VA to the high cervical ICA.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


