The high prevalence of unknown diabetes mellitus (DM) in patients with coronary disease and that the oral glucose tolerance test (OGTT) is the best diagnostic method in this context are well known. However, data about the incidence of DM in this population have not been well described. In the present study, we sought to determine the actual incidence of new-onset DM in patients with coronary disease using the OGTT. Our secondary objective was to validate a predictive model. We studied a series of 338 patients with coronary disease without known DM using the OGTT. After the OGTT, the patients were reclassified as normoglycemic, prediabetic, and unknown DM, according to the American Diabetes Association 2010 criteria. After 3 years of follow-up, the patients without DM were again reassessed using the OGTT. We then built a predictive model using the multivariate logistic regression method and validated it using the leave-one-out method. The final sample was 191 patients. The mean follow-up was 3.13 years. The overall incidence of DM was 43.6 cases/1,000 person-years (95% confidence interval [CI] 26.8 to 60.4). The incidence was significantly different between the initially normoglycemic patients (11.5%, 95% CI 2.3% to 31.8%) and the prediabetic patients (70.5%, 95% CI 42.7% to 98.3%; p <0.001). A risk model that included the glucose level 2 hours after challenge, glycosylated hemoglobin and triglyceride levels, and presence of noncoronary vascular disease showed good predictive capacity for incident DM (area under the curve 0.882, 95% CI 0.819 to 0.946; p <0.0001). In conclusion, the real incidence of new DM is very high in the coronary population, especially in those with prediabetes. It is necessary to use the OGTT for diagnosis, but we can optimize its indication using a risk model.
It is well known that diabetes mellitus (DM) is a potent risk factor for the development of cardiovascular disease. Moreover, morbidity and mortality increase when DM is associated with coronary heart disease. In part because of the increase in obesity, numerous studies have reported a progressive increase in the prevalence and incidence of DM in the general population worldwide. Although data within the coronary artery disease population subgroup have shown a high prevalence of known and unknown DM, less evidence has been reported of the real incidence of new cases of DM over time. In the coronary disease population, the recommendations for screening from the American and European societies differ. Although the European Diabetes Society has maintained its recommendation for systematic performance of the oral glucose tolerance test (OGTT) since 2007 (recommendation class I, evidence B), its American counterpart has recently rejected the routine use of this test owing to logistic criteria when making analytical determinations. The aim of the present study was to determine the actual incidence of DM in a cohort of patients with coronary disease using the OGTT. Second, to optimize OGTT use, we investigated the predictive factors for the development of DM.
Methods
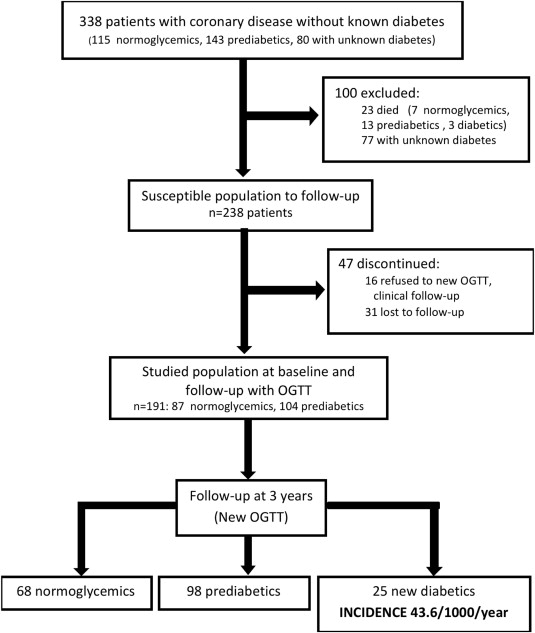
Our initial series has been previously reported. In brief, from November 2005 to May 2006, 580 consecutive patients underwent percutaneous coronary intervention. Of these 580 patients, 167 had a previous diagnosis of DM and 75 did not participate in the study for different reasons. Therefore, the final sample included 338 patients without known DM. These patients underwent the OGTT and were then reclassified according to the 2010 American Diabetes Association criteria as follows: 80 patients with unknown DM; 143, prediabetic; and 115, normoglycemic. In a follow-up visit after 3 years, these patients were reassessed with OGTT, excluding 147 patients (23 had died during this period, 77 had previously been diagnosed as having DM, and 47 refused to participate; Figure 1 ).
All patients underwent clinical follow-up examinations on an annual basis. We also included a physical examination, a lipid, renal, and hepatic profile, OGTT (1999 World Health Organization recommendation ), glycosylated hemoglobin (HbA1c), insulin and HOMA calculation at baseline and at 3 years of follow-up. Analyses were performed according to the usual practice at our biochemistry laboratory. HbA1c (ADAMS A1c, Nichols Institute Diagnostics, San Clemente, California) was measured using the Japanese method and converted to NGSP units with previously validated conversion equations using a computer system. The diagnosis of DM during the follow-up period was confirmed by the occurrence of any of the following criteria: (1) the diagnosis of DM by the primary care physician, (2) prescription of antidiabetic medication, (3) fasting glucose ≥126 mg/dl, (4) glucose level 2 hours after challenge ≥200 mg/dl, or (5) HbA1c ≥6.5%.
Continuous variables are presented as the mean ± SD. The Student-Welch test was used to check the equality between the mean values. Categorical variables are presented as the relative and absolute frequencies; 95% exact confidence intervals (CIs) were computed for the relevant percentages. Independence between categorical variables was checked using the exact Fisher test. The area under the receiver operating characteristic curves (AUCs) and odds ratios with their respective 95% CIs were used to measure the predictive capacity of each variable. Multivariate logistic regression analysis was used to build a predictive model. Afterward, a statistical weight was assigned for each predictive factor on the basis of the regression model β coefficients to develop a predictive scoring system. The AUC was also used to measure the predictive capacity of the proposed score, and a 95% bootstrap CI was likewise included. To validate the proposed model, a leave-one-out method was performed. p Values <0.05 were considered statistically significant.
Results
The distribution of the study population is shown in Figure 1 . Of the 338 patients, 23 died during follow-up (7 normoglycemic, 13 prediabetic, and 3 with unknown DM at baseline), with no relation evident between mortality and glycemic status (6.1% vs 9.1% vs 3.7%, respectively; p = 0.289). The average follow-up period was 3.13 years, and complete data (clinical and laboratory) were available for 191 patients (80.2% of the susceptible population). At baseline, 87 patients (45.5%) were normoglycemic and 104 (54.5%) were prediabetic. No differences were seen in the baseline characteristics between the patients with complete follow-up data and those lost to follow-up, except for the greater body mass index and greater frequency of metabolic syndrome in the former ( Table 1 ).
| Variable | Complete 3-y Follow-up | DM at 3-y Follow-up | ||||
|---|---|---|---|---|---|---|
| Yes (n = 191) | No (n = 47) | p Value | No (n = 166) | Yes (n = 25) | p Value | |
| Age (yrs) | 62.7 ± 12 | 65.3 ± 12 | 0.196 | 62.5 ± 13 | 64.3 ± 10 | 0.539 |
| Basal glycemia (mg/dl) | 96.0 ± 9 | 91.9 ± 10 | 0.057 | 94.6 ± 8 | 105.0 ± 11 | <0.001 |
| 2-Hour glycemia (mg/dl) | 136.0 ± 31 | 136.2 ± 33 | 0.968 | 131.4 ± 30 | 166.0 ± 23 | <0.001 |
| Triglycerides (mg/dl) | 128.6 ± 60 | 138.7 ± 101 | 0.840 | 124.4 ± 60 | 156.4 ± 56 | 0.001 |
| High-density lipoprotein (mg/dl) | 45.4 ± 11 | 47.6 ± 13 | 0.479 | 45.3 ± 12 | 46.0 ± 8 | 0.248 |
| Glycated hemoglobin (%) | 5.4 ± 0.3 | 5.4 ± 0.3 | 0.889 | 5.4 ± 0.3 | 5.6 ± 0.3 | 0.001 |
| Body mass index (kg/cm 2 ) | 29.0 ± 4 | 27.6 ± 3 | 0.015 | 29.0 ± 4 | 29.2 ± 4 | 0.520 |
| Men | 153 (80%) | 40 (85%) | 0.535 | 134 (81%) | 19 (76%) | 0.594 |
| Diabetes mellitus family history | 49 (26%) | 10 (22%) | 0.705 | 38 (23%) | 11 (44%) | 0.047 |
| Hypertension | 95 (50%) | 19 (40%) | 0.258 | 82 (49%) | 13 (54%) | 0.828 |
| Dyslipidemia | 92 (48%) | 21 (45%) | 0.745 | 84 (51%) | 8 (33%) | 0.113 |
| Smoker | 60 (32%) | 13 (28%) | 0.725 | 50 (30%) | 10 (42%) | 0.347 |
| Previous acute myocardial infarction | 64 (34%) | 21 (45%) | 0.176 | 56 (34%) | 8 (33%) | 1.000 |
| Vascular disease | 25 (13%) | 7 (15%) | 0.811 | 17 (10%) | 8 (32%) | 0.007 |
| β Blockers | 146 (76%) | 35 (76%) | 1.000 | 124 (75%) | 22 (88%) | 0.206 |
| Statins | 149 (78%) | 40 (87%) | 0.222 | 133 (80%) | 16 (64%) | 0.116 |
| Angiotensin-converting enzyme inhibitors | 69 (36%) | 17 (37%) | 1.000 | 56 (34%) | 13 (52%) | 0.116 |
| Diuretics | 24 (13%) | 4 (9%) | 0.614 | 18 (11%) | 6 (24%) | 0.098 |
| Metabolic syndrome | 76 (40%) | 10 (21%) | 0.018 | 59 (35%) | 17 (68%) | 0.004 |
The overall incidence of DM was 43.6 cases/1,000 person-years (95% CI 26.8 to 60.4). This incidence was significantly different statistically between the patients who had initially been normoglycemic (11.5 cases/1,000 person-years, 95% CI 2.3 to 31.8) compared with that (70.5 cases/1,000 person-years, 95% CI 42.7 to 98.3) for of those who had initially been prediabetic. The baseline characteristics of the patients who developed DM were different from those who did not ( Table 1 ). The former patients presented with greater values on the OGTT, greater HbA1c and triglyceride values, a greater prevalence of a family history of DM, and an increased incidence of associated vascular noncoronary disease.
A univariate analysis was performed to search for factors that predict the incidence of DM ( Table 2 ). The fasting glucose, glucose level 2 hours after challenge, HbA1c, and triglyceride values and the presence of vascular disease, metabolic syndrome, family history of DM, and previous treatment with β blockers were significantly associated with the incidence of DM. However, the last 2 factors did not have predictive capacity, because their AUC were not significantly different from 0.5.
| Factors | AUC (95% CI) | Cutoff ∗ | Sensitivity † | Specificity † | OR (95% CI) |
|---|---|---|---|---|---|
| Age (yrs) | 0.54 (0.42–0.66) | 67.7 | 0.48 | 0.64 | 1.01 (0.98–1.05) |
| Weight (kg) | 0.50 (0.37–0.63) | 81.3 | 0.52 | 0.61 | 0.99 (0.96–1.03) |
| Weight increase (kg) | 0.51 (0.39–0.63) | −1.65 | 0.79 | 0.32 | 0.99 (0.92–1.07) |
| Body mass index (kg/cm 2 ) | 0.54 (0.41–0.67) | 30 | 0.56 | 0.64 | 1.01 (0.91–1.11) |
| Abdominal perimeter (cm) | 0.56 (0.44–0.69) | 1.03 | 0.37 | 0.77 | 1.02 (0.97–1.07) |
| Metabolic syndrome | 0.66 (0.75–0.78) | — | 0.68 | 0.64 | 3.85 (1.57–9.46) |
| Systolic blood pressure (mm Hg) | 0.50 (0.36–0.63) | 167 | 0.12 | 0.96 | 1.01 (0.98–1.03) |
| Fasting glycemia (mg/dl) | 0.78 (0.67–0.89) | 102 | 0.64 | 0.85 | 7.44 (3.01–18.37) |
| 2-Hour glycemia (mg/dl) | 0.82 (0.74–0.90) | 140 | 0.84 | 0.67 | 8.92 (2.92–27.24) |
| Glycated hemoglobin (%) | 0.70 (0.61–0.80) | 5.5 | 0.78 | 0.56 | 5.32 (1.52–18.62) |
| Homeostasis model assessment | 0.57 (0.44–0.70) | 3.30 | 0.32 | 0.88 | 1.06 (0.92–1.22) |
| High-density lipoprotein (mg/dl) | 0.57 (0.46–0.68) | 43 | 0.72 | 0.51 | 1.01 (0.97–1.04) |
| Triglycerides (mg/dl) | 0.70 (0.60–0.79) | 104 | 0.88 | 0.49 | 6.82 (1.97–23.67) |
| Noncoronary vascular disease | 0.61 (0.48–0.74) | — | 0.90 | 0.32 | 4.12 (1.55–11.0) |
| Arterial hypertension | 0.52 (0.40–0.65) | — | 0.88 | 0.14 | 1.21 (0.51–2.86) |
| Family history of diabetes mellitus | 0.60 (0.48–0.73) | — | 0.90 | 0.22 | 2.63 (1.10–6.26) |
| β Blockers | 0.57 (0.45–0.68) | — | 0.88 | 0.23 | 2.48 (2.71–8.72) |
| Statins | 0.42 (0.29–0.55) | — | 0.64 | 0.20 | 0.44 (0.18–1.09) |
| Angiotensin-converting enzyme inhibitors | 0.59 (0.47–0.71) | — | 0.52 | 0.66 | 2.13 (0.91–4.97) |
| Diuretics | 0.57 (0.44–0.69) | — | 0.24 | 0.89 | 2.60 (0.92–7.35) |
∗ Value of each variable that reached maximum Youden index.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


