Fig. 3.1
Risk factors for radiotherapy toxicity
Interstitial fibrosis is common in the myocardium, from little lesions to very large diffuse infiltrates. The transition from type III collagen to type I increases myocardium stiffness and diastolic dysfunction. Fibrosis of conduction system explains the frequent occurrence of arrhythmias.
After 6 h from irradiation, an acute inflammation of small and medium arteries is present, with extensive neutrophil infiltration. After 2 days endothelial progressive destruction, vascular thrombosis, and reparative but insufficient attempts lead to myocardial ischemia and cell death. Progressively after 70 days, necrotic tissue is replaced by more or less extensive fibrosis.
ROS production is probably the first cause of endothelial damage, but other chronic inflammatory pathways are probably activated by irradiation. As a consequence, this mechanism leads to advanced and rapid atherosclerosis plaque formation and rupture.
Pericardial involvement is less frequent and intense than in the past when less developed techniques were used. Its characterization is an extensive fibrosis of the parietal pericardium, with possible adhesion and large fluid effusion. The increased permeability due to endothelium damage is the first moment of the radiotoxic effect on the pericardium, followed by ischemia and fibrosis. At this moment veins and lymphatics of the heart, pericardium, and mediastinum are deeply involved also, with a decreased draining capacity and a large recurrent fluid formation.
Enhanced anticipated fibrosis with calcification occurs on valves (mainly left-sided valves), with a not at all clear mechanism, probably due to the direct damage of the superficial endothelium of the valve itself.
Pathophysiology of coronary lesions seems to be an advanced quick atherosclerotic process that involves preferentially proximal ostial tracts, mainly the left main stem. The process is probably due to coronary endothelium lesions that enhance lipid permeability and inflammation of the plaque. The consequences are the possibility of plaque rupture and thrombosis. Moreover an endothelial damage may favor coronary spasms.
Also small and large vessels of cerebrovascular district are affected by irradiation of the brain, with advanced atherosclerotic damages and also alteration of permeability of the blood-brain barrier with direct effects on encephalic structures.
Neck irradiation can cause enhanced atherosclerotic lesions of carotid arteries.
As said before every structural and functional components of the heart can be the target of possible radiation damage. The pericardium, myocardium, valves, conduction system, and coronary arteries can be all affected although the thresholds and the way for injury differ slightly.
The radiation-induced damage pathophysiology has been studied extensively since the origin of the use of radiotherapy [3, 4].
3.3.1 Myocardium
A first common finding of myocardial injury is a marked, diffuse interstitial fibrosis, characterized by lesions with a diameter variable from a few millimeters to several centimeters that infrequently are involving all the entire myocardium and that can have a marked different distribution and severity between different regions.
From a histological point of view, there is not only an increase of the total magnitude of collagen but also a modification of the proportion of type I collagen that increases more than type III collagen. This modified proportion of the two types of collagen could be the cause of alteration of the compliance of the myocardium, contributing to and directly causing the diastolic dysfunction observed in the patients submitted to radiotherapy [5].
The radiation-induced myocardial fibrosis includes also the specialized myocardial cells of the conduction system. This finding is related and explains the findings of many reports of the occurrence of conduction defects and arrhythmias after chest radiotherapy.
The same findings explain the correlations between pathological and electrophysiological changes. One of the common pathophysiological pathways explaining cardiac damage seems to be damage at the level of myocardial microcirculation. As shown in the animal experiments on irradiated white rabbits by Stewart [6], the damages to the myocardium are developing in three phases. In the first 6 h after exposure to irradiation, an acute inflammation of the small and medium arteries appears, and a neutrophil infiltrate involving all layers of the heart is present. A second latent phase begins approximately after 2 days from the exposure to radiation, and it is possible to demonstrate only a relatively healthy pericardium and myocardium with a histological analysis, showing only modest but progressive fibrosis. However, the observation by means of electron microscopy of the myocardial endothelial cells reveals their progressive destruction leading to activation of reparative reactions with the obstruction of the lumen with fibrin and platelet thrombi. While the healthy endothelial cells try to react to the lesions beginning to replicate, often the rate of production of new capillaries is insufficient, so the reduction in microvascular functioning structures can cause ischemia and can lead to myocardial cell death with successive fibrosis. The third late phase occurs about 70 days after irradiation when many animals die. In this stage the common typical histologic signs are that of a marked and extensive myocardial fibrosis. In this experimental setting, the schedule of administration of radiation given to these rabbits is not at all the same as in patients undergoing radiotherapy; however, the gross and microscopic myocardial alterations shown in rabbits during the latent and late stages are similar to those observed in man histological samples, suggesting a similar pathogenesis. It is a matter of debate whether free radicals produced by radiation are the first cause of the endothelial and myocardial damage. Effectively, cells respond to radiation injury increasing ROS production probably through activation of mitochondrion-dependent and mitochondrion-independent metabolic enzymes, including nitric oxide synthesis and oxidoreductase enzymes. This activation seems to create a favorable environment for the occurrence of other types of injury caused by oxidative damage to proteins and lipids [7].
As discussed above, radiation can activate other inflammatory pathways. These pathways may be relevant in cells exposed to radiation, because they can lead to chronic inflammation, enhancing as a consequence the risk of clinically important atherosclerotic plaque development and rupture with the clinical development of acute MI and ischemic stroke, as demonstrated for the development of atherosclerosis in the general population.
The observations of Japanese atomic bomb survivors exposed to body irradiation at doses not particularly elevated have shown a possible dose-dependent chronic inflammation due to radiation exposure [8].
Also recent studies demonstrated, even for irradiation of low level in young children, a relative increase in cardiovascular morbidity and mortality, related to high levels of different markers of inflammation like C-reactive protein. The same results were observed in a longitudinal cohort study by Lipsultz [9] on children ≥3 years after cancer therapy (Fig. 3.2).
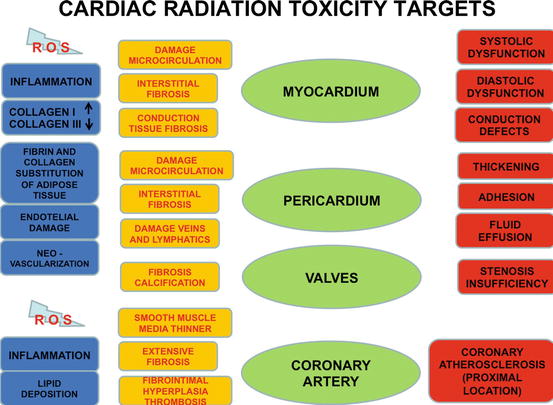

Fig. 3.2
Possible targets of radiotherapy toxicity
3.3.2 Pericardium
The involvement of pericardial structures occurred in the past with older radiotherapy techniques. Although the high doses and not highly developed radiotherapy techniques were used for the past 25 years in children, with the recent progress in the field, this event has now become rare [10]. The effects of irradiation lead to extensive damage including extensive fibrous thickening of the pericardium, pericardial adhesions, and increase of pericardial fluid. The parietal pericardium generally is more frequently, severely, and extensively involved than the epicardial pericardium. Microscopically the replacement of the normal pericardial adipose tissue with fibrin and thick collagen can be shown. Fibrosis in the stroma and on the mesothelium surfaces may occur. When pericarditis develops as shown in the myocardium, the alteration in the function of the endothelial cells increases their permeability and deeply damages their function, even though a certain proliferation of small blood vessels throughout the irradiated pericardium may be demonstrated. This alteration of pericardial vascular net causes ischemia and eventually fibrosis. Associated to this, a certain grade of fibrosis of the venous and lymphatic channels of the heart, of the pericardium, and of the mediastinum is generally identified that decreases the possibility of draining extracellular fluid. These two mechanisms can together cause the accumulation of a protein-rich effusion in the pericardial space [11]. Fibrin contained in this effusion may also be a product of intense fibrinolysis.
3.3.3 Valves
The effect of irradiation on cardiac valves may consist of an enhanced and anticipated fibrosis, with or without calcification [10, 12]. Due to the fact that heart valves are not vascularized, the pathophysiology of these valve changes is difficult to explain, but for sure it cannot be explained by vascular damage. It could be hypothesized that the fibrosis is the result of a late injury to the endothelium that covers their structures. Commonly the damages seen on aortic and mitral valves are prevalent and more severe than that observed to tricuspid and pulmonary valves. This effect is not related to the dose distribution, and the pathogenesis of the lesions seems to be rather a consequence of the higher pressures of the left heart. Otherwise in at least five patients but at doses >40 Gy, valve stenosis and fibrosis of the whole pulmonary outflow tract have been reported [13].
3.3.4 Coronary Arteries
The pathology and pathophysiology of the coronary lesions after radiotherapy seems to be similar to that of coronary atherosclerosis seen in the general population. The similarity regards both the location and the morphology of the coronary lesions. Left anterior descending and right coronary arteries are the most commonly involved after radiotherapy [14, 15], but an increase of the disease of the left main coronary artery in patients exposed to chest radiotherapy in comparison to patients with typical CAD has been shown. It was hypothesized that this higher number of lesions of the main left branch could be due to the old technique of anterior-weighted irradiation. Generally the lesions in the diseased vessels occur in the proximal tract and often involve the coronary ostia [10, 12, 14, 15].
Histologic samples of 16 patients treated with radiotherapy and with radiation-associated heart disease found that the smooth muscle layer in the media tended to be thinner than that of ten control subjects with typical CAD. In the same samples, the media and adventitia were also more thickened with fibrous tissue than that of generic coronary lesions. Many investigators found that intimal plaques are largely composed of fibrous tissues, with a little lipid component. This finding remains controversial. Other investigators have found atherosclerotic plaques with rich lipid content, associated with a large fibrotic reaction [12]. Although these features suggest disease caused by radiation, like the proximal location of the plaque and the replacement of smooth muscle with extensive fibrosis, the histological discrimination in any particular case of a radiation-induced lesion from typical atherosclerosis may be difficult.
It is also difficult to understand the extent to which the pathophysiology of radiation-induced CAD differs from that of typical CAD. After irradiation, also the endothelium of coronary artery is probably damaged with a mechanism similar to that damaging the endothelium of the microvasculature of the myocardium, producing fibro-intimal hyperplasia and leading to possible thrombus formation and to lipid deposition, with an evolution resembling that of the typical mechanism of CAD. However, there are conflicting data from animal studies on radiation-associated coronary disease regarding lipid deposition. While in rabbits a high-fat diet was necessary for the occurrence of atherosclerotic lesions after irradiation, in dogs even with a normal diet, typical atherosclerotic plaques have been observed. On the other hand, these and other studies on animals are agreeing on the observation that a high-fat diet over radiation accelerates atherosclerosis [16–21].
Finally, in some cases of sudden cardiac death, coronary artery spasm has been suggested in a single case report.
3.3.5 Cerebrovascular Disease
The pathophysiological effects of human cerebrovascular radiation come from in vitro or animal models using non-fractionated, supratherapeutic radiation [22]. The histological and cellular modifications of the human cerebral vascularization to radiotherapy can be characterized in relation to vessel diameter and time from treatment.
Also in the cerebral district, the smallest arteries, arterioles, and capillaries are the structures most vulnerable to radiation.
Endothelial cell damage creates an inflammatory response, causing endothelial proliferation with increased platelet adherence and thrombus formation [23].
In the brain, loss of tight junctions and increased vesicular activity of the blood-brain barrier increase vascular permeability.
After a long time from the exposition, the vascular density of the cerebral tissue decreases. In the large vessels of the central nervous system, the muscular tunica of the media is thicker and more resistant to radiation effect, but the endothelium may be damaged in these larger vessels as in the other [24]. As a consequence, histopathologic modifications similar to advanced atherosclerosis may occur with luminal narrowing and thrombus formation and later to abnormal dilatation and tortuosity.
Radiation therapy of the circle of Willis may lead to similar cerebrovascular abnormalities. A complete occlusion of ≥1 of the three major cerebral vessels may lead to the development of small, collateral vessels, in the attempt to maintain a sufficient vascularization of the brain. Such patients are at increased risk for cerebrovascular events.
3.3.6 Carotid Artery Disease
In the first year after neck irradiation, a certain degree of increase in the thickness of the carotid wall that may be associated with an increased risk of stroke may be shown.
Preexisting atherosclerotic lesions at time of treatment can act as an exacerbating factor. As for advanced atherosclerosis of other vascular districts, the common risk factors as hypertension, obesity, smoking, diabetes mellitus, and other known associations with both cardiac and cerebrovascular disease in the general population are further worsening the damage of irradiation.
Box 3.1: Radiotherapy: General Principles
Radiation damage slowly progressive
All cardiac anatomic structures involved
Clinic expression complex and often combined
Relative risk of mortality from 2 to 7 for Hodgkin’s disease and from 1 to 2.2 for left breast cancer
Risk higher in younger patients
Risk related to overall dose, dose per fraction, volume of heart irradiated, age at exposure, time from exposure, concomitant chemotherapy, cardiovascular risk factors
3.4 Clinical Expressions of Radiotherapy Cardiac Toxicity
Lesions of different cardiovascular structures may occur very often in a contemporary time and with difficult clinical conditions that may overlap.
Acute pericarditis with typical chest pain, fever, and ST-T changes and large effusions that can lead to tamponade may develop after 2–145 months. Its incidence in the past reached around 40 % of treated patients, while now is limited to 2–5 %.
A chronic pericardial effusion may develop after 6–12 months from treatment, and it is to take into account the differential diagnosis with a neoplastic recurrence.
Spontaneous resolution is the rule, but it can evolve in chronic constrictive pericarditis in around 20 % of cases also after 10 years from the treatment.
Advanced coronary artery disease evolves after 10–15 years (mean 82 months), often in a completely nonsymptomatic mode, and the first expression may be an acute myocardial infarction or sudden death. Therefore an early diagnosis is mandatory with a careful investigation, also after many years from treatment, with all diagnostic method commonly used.
The treatment is similar to that of common coronary disease, but a surgical approach can be very difficult due to extensive mediastinal fibrosis.
Left-sided valve disease may be evident after 10 years from irradiation, with some expression of advanced disease. Also in this case, surgical repair may be difficult for mediastinal fibrosis.
Heart failure may be due to direct myocardial damage and to coronary and valve disease and may lead to differential diagnosis with chronic pericarditis. It can evolve over a large time interval, also after many years from treatment.
Conduction tissue involvement may lead to a typical sick sinus syndrome, intraventricular blocks, and atrioventricular blocks often of progressive worsening degree, requiring pacemaker stimulation. Fibrosis may also be the anatomic substrate of reentrant supraventricular and ventricular arrhythmias.
It is noteworthy that also devices may be damaged from high-energy irradiation, and these procedures are to be integrated by careful monitoring of their electronic functions.
Also the heart’s nervous system may be damaged with the possibility to develop an unappropriated sinus tachycardia, a heart rate variability imbalance, and an enhanced pain threshold that explains the reduced incidence of overt angina also in the presence of severe coronary lesions.
Finally mediastinum irradiation may lead to pulmonary fibrosis and consequent pulmonary arterial hypertension and to hypothyroidism secondary to gland damage.
The injury of the various structures and tissues of the heart can cause a spectrum of radiation-induced CV diseases that often can overlap each other.
The late effects, hereafter discussed, generally occur with radiation tolerance doses not exceeding 30–40 Gy.
A pericardial disease after radiation therapy most commonly presents as a pericardial effusion or typical pericarditis with the frequent and extensive involvement of the right ventricle [2, 12]. The time between radiation therapy and symptom development is variable, ranging from 2 to 145 months.
Pericardial effusion is typically an early presentation: acute symptomatic pericarditis with hemodynamic malfunction secondary to large pericardial effusion leading to tamponade can develop in the first times after irradiation, and commonly it is the first clinical expression of the disease of cardiac toxicity of radiotherapy. Around 40 % of patients submitted to radiotherapy with antiquated techniques could develop pericarditis, while with the modern techniques utilizing a total dose <30 Gy, subcarinal blocking, and a fractioned daily dose <2 Gy, the incidence is decreased to about 2.5 % (from 2 to 5 %). The clinical findings of this picture are the same as an acute pericarditis of other etiology. Symptoms can begin with the appearance of the typical pericardial pain with fever and the typical electrocardiographic findings of ST-T segment changes and of reduction of amplitude of the QRS voltage. Also chest X-ray may be helpful showing an enlargement of the cardiac shape related to the amount of pericardial effusion. A picture of asymptomatic chronic pericardial effusion may generally develop usually 6–12 months following RT.
In the case of the occurrence of a pericardial effusion, the differential diagnosis of the condition is mandatory, particularly with a possible neoplasm involvement of the pericardium.
Normally the evolution of a pericardial effusion is the spontaneous resolution of the picture, but in around 20 % of patients, a chronic and/or constrictive pericarditis may develop also after 10 years from the end of the treatment, usually appearing not before 18 months.
Arteritis of the coronary arteries and accelerated atherosclerosis can cause premature advanced coronary artery disease particularly of the first tract of left anterior descending and right coronary artery and also of the main left stem (Fig. 3.3).
The incidence of coronary accelerated atherosclerosis is difficult to evaluate, but it is frequently shown also in patients without the traditional risk factors. The appearance of clinical consequences on coronary circulation can occur also 10–15 years after RT; however, the mean interval for the development of CAD after radiation therapy shown in a series of patient was approximately 82 months.
The clinical characteristics of a coronary disease due to irradiation may vary from a completely asymptomatic picture to that of an acute myocardial infarction and also to sudden death without previous expressions. Indeed, around 50 % of asymptomatic patients may develop new myocardial perfusion defects [10, 25, 26]. When symptoms are present, most patients experience angina, dyspnea, or heart failure. Sudden death can be the first sign of the disease and generally is the result of diffuse intimal hyperplasia of all main coronary arteries or of left main stenosis. Sometimes, coronary spasm may develop in patients with otherwise normal appearing coronary vessels.
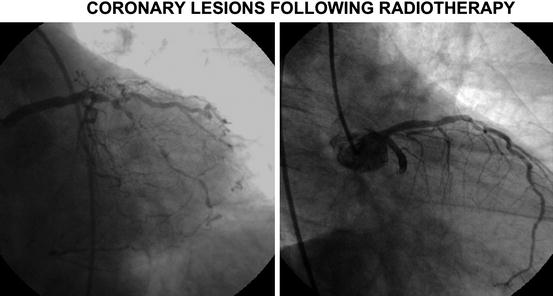

Fig. 3.3
Coronary disease due to radiotherapy toxicity. (a) Main left stem stenosis in a 54-year-old patient presenting with effort dyspnea. Hodgkin’s lymphoma treated with thoracic radiotherapy at the age of 26 years. (b) Proximal circumflex stenosis in a 51-year-old female with AMI treated with radiotherapy for left breast cancer at the age of 38 years
The diagnosis of a coronary complication from radiotherapy toxicity may be difficult before its display, and it has to be carefully investigated in patients at risk, even many years after irradiation. Management of radiation-induced CAD is similar to that of atherosclerotic disease. Both percutaneous intervention and coronary artery bypass grafting have been used [25]. Surgical bypass grafting may be more difficult in patients with radiation-induced atherosclerosis because of mediastinal fibrosis, which is associated with a high incidence of complications.
Valve stenosis and regurgitation of mitral and aortic valves are the common expression of radiation damages to heart valves, while very uncommon are damages on the right heart valves. This kind of radiation toxicity is commonly seen not before 10 years from the end of the treatment, and it is generally associated to pericardial and other structure damage. However, the data of a patients’ series with valve involvement after radiotherapy demonstrate a mean time from irradiation to onset of symptoms of approximately 98 months [10]. Only a minority of patients seem to develop clinically moderate or severe valve dysfunction (Fig. 3.4).
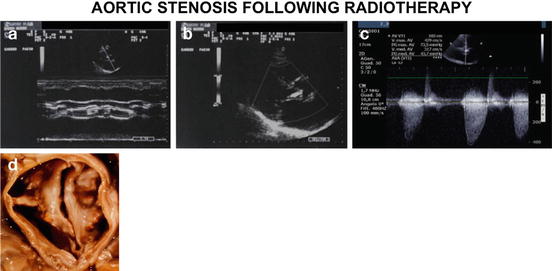

Fig. 3.4
Valve heart disease due to radiotherapy toxicity. Severe aortic stenosis in a 73-year-old man with radiotherapy for a Hodgkin’s lymphoma at age 58. In (a, b) monodimensional and bidimensional echocardiogram; in (c) Doppler velocity. Valvular gradient max 118 mmHg, mean 68 mmHg; anatomical valvular area 0.6 mm2. In (d) anatomical specimen during surgical operation of valve replacement
Heart valve replacement is often difficult for possible surgical complications due to mediastinal and pericardial fibrosis, to the frequent development of conduction defects requiring permanent stimulation, and to the association of coronary lesions that can be present in around 23 % of the patients.
A clinical picture of chronic heart failure can appear during time, also after many years, as a consequence of a cardiomyopathy characterized to nonspecific diffuse interstitial fibrosis and myocarditis, also in association with the other radiation toxicity effects on valve and on coronary vessels. The common myocardial toxicity expression is a picture of diastolic dysfunction. A systolic dysfunction is generally seen in patients that have been submitted to chemotherapy, mainly with anthracyclines. The commonest clinical finding if the diastolic dysfunction become manifest is that of a restrictive cardiomyopathy that sometimes can be difficult to distinguish from the picture of a chronic pericarditis.
Fibrosis of the conduction system can lead to all the possible pictures of altered formation of the impulse or conduction defects. The clinical pictures vary from supraventricular and ventricular premature beats to complex ventricular arrhythmias, typical sick sinus syndrome, or atrioventricular and intraventricular complete or incomplete heart block that may evolve in their complexity during time and that may require the implantation of a pacemaker. Right bundle branch seems to be more common than left bundle branch block. Conduction defects are generally present for doses higher than 40 Gy and may evolve also after more than 10 years after the end of radiotherapy. Predisposing factors seem to be previous conduction defects like right BBB and the contemporary presence of a pericardial or valve complication.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


