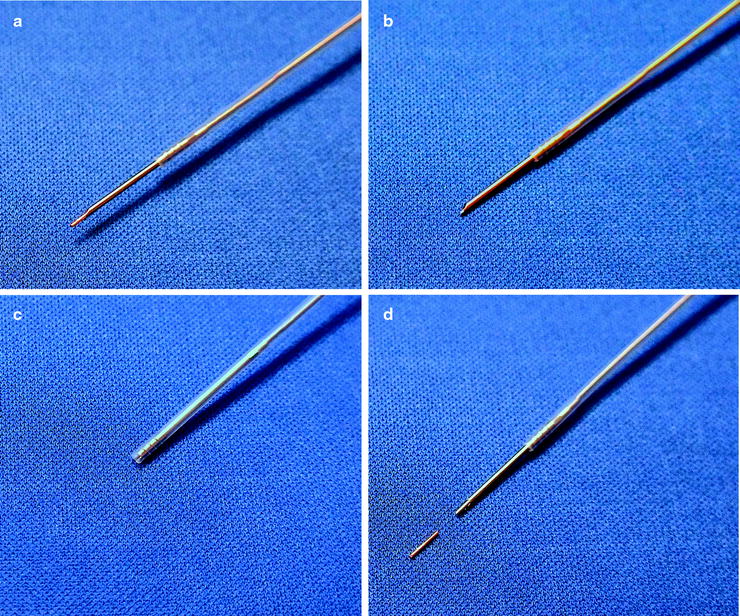
Fig. 39.1
Generic margin expansion to accommodate microscopic tumor extension beyond the radiographic borders of the tumor, errors that occur in daily patient positioning, and tumor movement secondary to respiratory motion. Panel A shows a generic tumor. The lightly dashed line represents the margin expansion added to increase the volume of planned radiation delivery to accommodate microscopic tumor extension beyond the radiographic borders of the tumor. Panel B shows the same generic tumor with its expanded margin as it might appear on three separate days. The variability in position is related to errors in daily patient positioning. The heavy dashed line represents the margin expansion performed to accommodate the both microscopic tumor and daily patient positioning errors. Panel C shows the same generic tumor with its expanded margins as it might move during respiration. The solid line represents the margin expansion performed to accommodate microscopic tumor, daily patient positioning and respiratory motion. As can be seen, the volume of tissue treated has the potential to be significantly larger than the actual volume of the tumor
To take full advantage of the tightly focused and conformed radiation delivery volume, it is necessary to know where the tumor is actually located from day to day and breath to breath. Since direct visualization of the tumor is not possible while the X-ray beam is actually on, and even when the beam is off, it is often difficult to visualize the tumor on fluoroscopy, surrogate markers that can be easily seen of continuously tracked can be placed in or near the tumor target. These surrogates are called fiducials; their use, variety, implantation, and the reported experience with them are discussed in the following.
Fiducial Markers and Their Use
A fiducial marker, or fiducial, is a small object placed on or in the body to mark an area for subsequent diagnosis or treatment. In the field of radiation therapy, internally implanted fiducials are used as localizable and trackable surrogates of tumor location. Their value arises from the fact that many soft-tissue tumors are difficult to visualize on fluoroscopy. And even on CT scan images when it is easy for the human eye to identify the location and extent of the tumor, it remains very difficult for automated computer hardware and software to do this same identification. To overcome this, radiopaque metallic fiducials provide very high contrast on both fluoroscopy and CT scan images and are therefore easier to detect by both the human eye and the automated computer hardware and software. The position of these fiducials can be determined at the time of setup prior to the initiation of radiation delivery, and even during the treatment session, their position can be determined by intermittently turning the radiation beam off.
Alternatively, some markers act as wireless electromagnetic transponders (Beacon® Transponders, Calypso Medical Technologies Inc., Seattle, WA, USA) and are radiopaque and can also be localized and tracked in a manner analogous to that used for electromagnetic navigational bronchoscopy. These sorts of markers can be localized using the same methods and with the same limitations experienced with standard fiducial markers but also be localized and tracked by dedicated equipment while the radiation beam is on.
Fiducials potentially allow a more focused delivery of radiation with narrower treatment margins to be used. This can be accomplished by combining a method of conforming and focusing the radiation beam to the size and shape of the tumor with (1) improved patient setup, (2) respiratory gaiting, and (3) real-time tumor-tracking radiation therapy.
Daily errors in patient setup occur because the patient is not placed in the exact same position relative to the linac every day. By more accurately knowing the location of the tumor target on the day of treatment, the margin expansion to accommodate errors in patient setup (the margin expansion from panel A to panel B in Fig. 39.1) can be decreased.
Respiratory gating is a method of turning the radiation beam on when the tumor target is located at the focal point of the radiation beam and it is turned off as soon as the target moves outside of the beam. The patient is typically aligned so that the beam is on during expiration. Such gating also allows the margin expansion to accommodate respiratory motion (the margin expansion from panel B to panel C in Fig. 39.1) to be decreased.
Real-time tumor tracking is a method of dynamically moving the focal point of the radiation mean so that this focal point always corresponds with the position of the tumor target. Such gating allows the margin expansion to accommodate respiratory motion (the margin expansion from panel B to panel C in Fig. 39.1) to be decreased.
While a variety of radiation therapy systems are capable of real-time tumor tracking, based on the published literature, the system most widely used in conjunction with fiducials is the CyberKnife® (Accuray, Sunnyvale, CA) system. The CyberKnife (Fig. 39.2 panel A and B) consists of a small 6-MV linac mounted on a computer-controlled robotic arm capable of moving with 6° of freedom, two orthogonally placed X-ray, two optical cameras, and an optic-radiographic motion monitoring system for real-time tumor tracking. Radiopaque fiducial markers implanted in or near the tumor and light-emitting diodes placed on the patient’s chest are used for target tracking. Prior to radiation treatment and while the X-ray beam is off, a series of X-ray images are taken by the two orthogonal cameras at various times during the respiratory cycle and the system develops a mathematical model relating the locations of the light-emitting diodes on the chest with those of the fiducials near the tumor in the lung. Continuous tracking of the light-emitting diodes combined with this mathematical model allows the robotic arm to be moved in real time such that the radiation beam tracks the moving tumor target. At regular intervals during treatment, additional orthogonal X-ray images are taken to allow the model to be validated and updated with small changes in breathing patterns. The total system accuracy of the device and the respiratory motion tracking has been reported as <1 mm. The current system allows for a dozen different beam directions from over a 100 different robot arm locations, providing more than 1,200 possible beam paths.
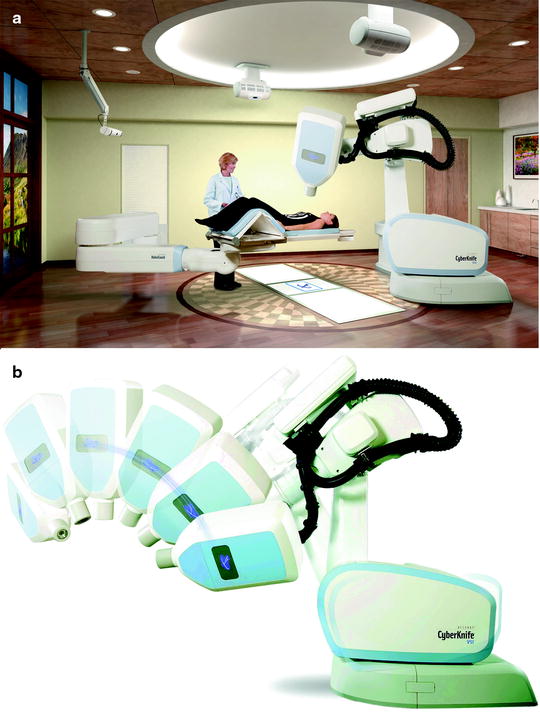

Fig. 39.2
The CyberKnife system consists of a small 6-MV linac mounted on a computer-controlled robotic arm capable of moving with 6° of freedom, two orthogonally placed X-ray, two optical cameras, and an optic-radiographic motion monitoring system for real-time tumor tracking. Radio-opaque fiducial markers implanted in or near the tumor and light-emitting diodes placed on the patient’s chest are used for target tracking. Prior to radiation treatment and while the X-ray beam is off, a series of X-ray images are taken by the two orthogonal cameras at various times during the respiratory cycle and the system develops a mathematical model relating the locations of the light-emitting diodes on the chest with those of the fiducials near the tumor in the lung. Continuous tracking of the light-emitting diodes combined with this mathematical model allows the robotic arm to be moved in real time such that the radiation beam tracks the moving tumor target. At regular intervals during treatment, additional orthogonal X-ray images are taken to allow the model to be validated and updated with small changes in breathing patterns. The current system allows for a dozen different beam directions from over a hundred different robot arm locations, providing more than 1,200 possible beam paths. Panel A shows an overview of the Cyberknife System with a patient positioned on the couch. Panel B shows demonstrates the motion capabilities of the Cyberknife linac (Images used by permission of Accuray Incorporated)
Desired Positioning and Implantation Planning
The goal of fiducial marker implantation is to provide enough useful and accurate information to the radiation oncologist to make the added effort and risk worthwhile for the patient. There are several goals to consider when deciding the number and location of fiducial implantation.
When considering the number of fiducials to implant, enough fiducials should be place to provide accurate positional information and ideally rotational information as well. Two fiducials located in and around the tumor such that they bracket the bulk of the tumor can provide translational information about the tumor target. Three fiducials located in and around the tumor such that they bracket the bulk of the tumor and do not overlap on the fluoroscopic tracking system can provide both translational and rotational information. Additionally, enough fiducials should be placed so that it is possible to tell if a fiducial has migrated out of position and ideally enough so that you can tell which of the fiducials have migrated. Migration can be detected by simply measuring the distance between pairs of fiducials at the same phase of respiration and if a significant change in this distance has occurred, then one of the two fiducials has migrated. When only two fiducials have been implanted, it is possible to tell that one of the fiducials has moved, but not which one. When three or more fiducials have been implanted, by measuring the distance between each fiducial and all the other fiducials, it is possible to tell not only that one of the fiducials has migrated but which one. This fiducial can be ignored and positional and tracking information obtained from the remaining fiducials.
When considering where to implant the fiducials, they should be close enough to the tumor target so that they move with the tumor and provide accurate information about the tumors location and motion. They should be far enough away from each other so that each fiducial provides independent information; fiducials implanted very close to one another look like a single fiducial on fluoroscopy. They should not be so far apart that they are outside the field of the view of the tracking system. They should not be positioned such that a straight line can be drawn between any three of the fiducials; such collinear fiducials may appear superimposed on fluoroscopy and thus will not provide useful information. Representative positioning of a group of fiducial markers is shown in Fig. 39.3.
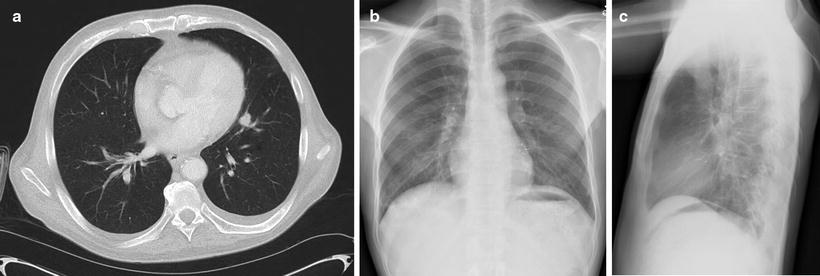

Fig. 39.3
Recommended placement of fiducial markers near a small left sided tumor. Panel A shows a cross sectional CT image of the target tumor. Panel B and C show a posterior-anterior and lateral chest X-ray of the target tumor following implantation of four 0.75 mm diameter coiled wire fiducials in and around the tumor. Note that the fiducials are not collinear on either orthogonal image and while the tumor is not clearly visualized, the fiducials are located such that they bracket and surround the tumor. Also note the close proximity of the tumor to the heart, which makes the tumor very difficult to visualize tumor on these X-ray images
Specific recommendations for fiducial implantation are as follows:
With currently available fiducials, 4–6 should be implanted to so that three remain in place. Should fiducials with less likelihood of migration become available, less could be implanted.
Fiducials should be placed in and around the tumor, such that the bulk of the tumor is bracketed by the fiducials.
Those fiducials placed outside the tumor are placed as close to the edge of the tumor as possible and ideally in the same lobe as the tumor. The minimum distance from the edge of the tumor is not known.
There should be a minimum spatial separation between adjacent fiducials of 1.0–2.0 cm.
There should be a maximum spatial separation between any two fiducials of approximately 7–10 cm depending on the tracking system.
Fiducials should not be placed collinearly or positioned such that a straight line can be drawn through any three.
With these recommendations as a guide, planning of the desired implantation sites proceeds in a manner very similar to planning for a biopsy. Unlike planning for a biopsy where the center of the tumor or lymph node is typically chosen as the target site with the intent to obtain a specimen from the tumor itself, the desired implantation site is often adjacent to the tumor and may actually be a several centimeters away from the center or even the edge of the tumor. This is particularly true for fiducials implanted bronchoscopically into small airways in or near the tumor where the most desirable airway may be some distance from the tumor.
Types of Fiducial Markers
Commercially available fiducial for use in marking lung tumors can be categorized in two basic types: (1) those intended for implantation directly into the tumor or into the lung parenchyma near the tumor and (2) those intended for implantation in small-diameter airways near a tumor. A wide variety of these markers of are commercially available and they are presented in Table 39.1.
Table 39.1
Summary of fiducials commonly used in the lung: outlining their shapes, sizes, preferred implantation methods and locations, and sources
Shape | Material | Diameter (mm) | Length (mm) | Surface | Trade name | Source |
|---|---|---|---|---|---|---|
Implantation into the tumor or surrounding parenchyma with a needle | ||||||
Coiled wire | Gold | 0.35 | 20 | Ribbed | Visicoil™ | Core Oncology, Santa Barbara, CA, USA |
Cylindrical | Gold | 0.45 | 10, 20 | Stepped cylinder | X-mark™ | Onc Solutions, Acton, MA, USA |
Coiled wire | Gold | 0.5 | 20 | Ribbed | Visicoil™ | Visicoil, Core Oncology, Santa Barbara, CA, USA |
Implantation into the tumor or surround parenchyma with a needle or into small airways near or in the tumor with a catheter | ||||||
Coiled wire | Gold | 0.75 | 20 | Ribbed | Visicoil™ | Visicoil, Core Oncology, Santa Barbara, CA, USA |
Cylindrical | Gold | 0.8 | 3.0, 5.0 | Smooth | Gold fiducial marker | Stellar Medical, Fort Meyers, FL, USA |
Cylindrical | Gold | 0.8 | 3.0 | Knurled | Standard soft tissue | Civco, Orange City, IA, USA |
Cylindrical | Gold | 0.85 | 5.0, 10, 20 | Stepped cylinder | X-Seed™, X-Mark™ | Onc Solutions, Acton, MA, USA |
Stellar Medical, Fort Meyers, FLA, USA | ||||||
Cylindrical | Gold | 0.9 | 3.0 | Knurled | Standard soft tissue | Civco, Orange City, IA, USA |
Coiled wire | Gold | 0.9 | 10, 20 | Ribbed | FlexiCoil™ | Civco, Orange City, IA, USA |
Cylindrical | Gold | 1.0 | 3.0, 5.0 | Smooth | Gold fiducial marker | Stellar Medical, Fort Meyers, FL, USA |
3.0, 10 | Gold seed | Onc Solutions, Acton, MA, USA | ||||
Implantation into small airways near or in the tumor with a catheter | ||||||
Coiled wire | Gold | 1.1 | 20 | Ribbed | Visicoil™ | Visicoil, Core Oncology, Santa Barbara, CA, USA |
Cylindrical | Gold | 1.15 | 5.0, 10, 20 | Stepped cylinder | X-Seed™, X-Mark™ | Onc Solutions, Acton, MA, USA |
Stellar Medical, Fort Meyers, FL, USA | ||||||
Cylindrical | Gold | 1.2 | 3.0, 5.0 | Smooth | Gold fiducial marker | Stellar Medical, Fort Meyers, FL, USA |
3.0, 10 | Gold seed | Onc Solutions, Acton, MA, USA | ||||
Cylindrical | Gold | 1.2 | 3.0 | Knurled | Standard soft tissue | Civco, Orange City, IA, USA |
Coiled wire | Gold | 1.2 | 10, 20 | Ribbed | FlexiCoil™ | Civco, Orange City, IA, USA |
Cylindrical | Gold | 1.6 | 3.0 | Knurled | Standard soft tissue | Civco, Orange City, IA, USA |
Cylindrical | Metal and glass | 1.8 | 8.5 | Smooth | Beacon® transponder | Calypso Medical Technologies, Inc., Seattle, WA, USA |
Sphere | Gold | 2.0 | – | Smooth | Bone marker | Civco, Orange City, IA, USA |
Fiducials for implantation into the tumor or lung parenchyma offer the greatest versatility in use. However, care must be taken during insertion to avoid implantation into pulmonary vasculature. In their simplest form, they are small-diameter metallic cylinders with typical diameters of 0.8 mm and lengths of 3.0–5.0 mm. Small-diameter coiled metallic wires are also available with useful diameters of 0.35–0.75 mm and lengths of 10–20 mm. They can be placed in mediastinal lymph nodes, central tumors, or peripheral tumors. They can be implanted percutaneously with a relatively small-bore aspiration needle and guided with computed tomography, fluoroscopy, or ultrasound. They can also be implanted bronchoscopically using an aspiration or biopsy needle passed through the working channel of the bronchoscope, guide sheath, or extended working channel.
Fiducials for implantation into small airways near a tumor are limited to use for tumors with small-diameter airways nearby, although it is possible in more friable tumors to implant these directly into the body of the tumor. In their simplest form, they are metallic cylinders with typical diameters of 0.8–1.6 mm and lengths of 3.0–5.0 mm or 2.0-mm-diameter metallic spheres. Some of the cylinders may have a roughened “knurled” surface, or a diameter that abruptly changes along its length that produces a “stepped” appearance on longitudinal cross section; these shapes are intended to improve stability in when implanted in tissue or an airway. Coiled metallic wires are also available with useful diameters of 0.75–1.2 mm and lengths of 10–20 mm. Implantation is done bronchoscopically with guidance provided by fluoroscopy, electromagnetic navigation, or radial ultrasound.
Transthoracic Method of Fiducial Implantation
Placement of fiducial markers using a transthoracic method is very similar to performance of a transthoracic needle aspiration or biopsy. Following review of the pertinent imaging and localization of the tumor target, the patient is positioned on the procedure table such that the target may be directly and readily accessed through the chest wall. Imaging of the chest with either computed tomography, fluoroscopy, or ultrasound is repeated to again identify the target and to select the proper site for insertion of the needle. The site should be prepped and draped using standard sterile technique. Topical anesthesia is provided to the selected area, with special attention paid to the skin surface, the superior margin of the inferior rib, and the parietal pleura. A fiducial marker preloaded in an introducer needle is selected. Preference to smaller diameter needles is given because they potentially cause lower rates of pneumothorax. A variety of fiducial shapes and sizes are available and come in preloaded needle from most of the manufacturers listed in Table 39.1. The needle is then passed though the anesthetized area and into the desired location in the target tumor or the adjacent lung parenchyma. The needle trocar is then held in place and the needle withdrawn, thereby depositing the fiducial marker in the tumor or lung. Transthoracic implantation of fiducial marker into a peripheral lung nodule using fluoroscopic guidance is shown in Fig. 39.4.
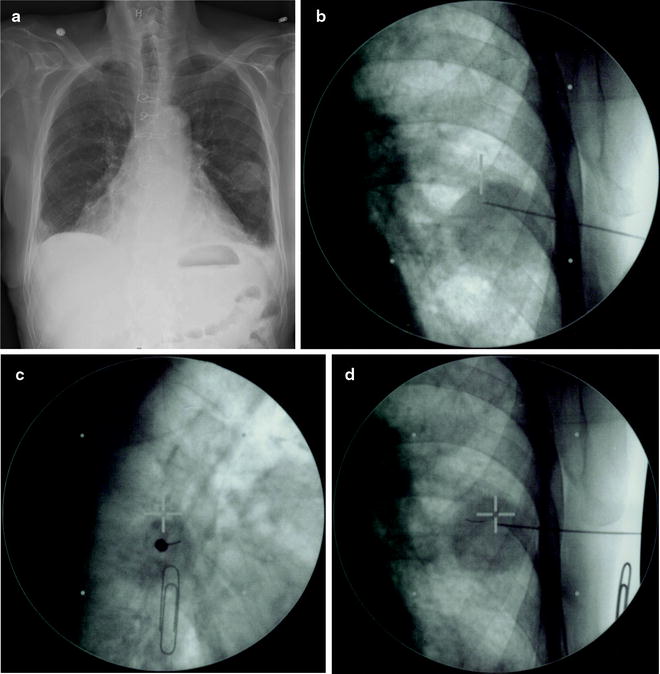

Fig. 39.4
This figure demonstrates percutaneous placement of a fiducial marker in a peripheral pulmonary tumor in the left lung. Panel A is a posterior-anterior chest X-ray of the tumor target. Panel B shows a posterior-anterior fluoroscopy image of a 18-gauge needle pre-loaded with a 0.75 mm diameter coiled wire fiducial as it is advanced into the tumor target. Panel C shows an orthogonal fluoroscopy image of the same 18-gauge needle as it is advanced into the tumor target. Such orthogonal fluoroscopy images allow more accurate positioning of the fiducials in and around the tumor both with this percutaneous approach and when implantation is performed bronchoscopically. Panel D shows the fiducial (to the lower left of the cross-hairs) in the tumor following deployment
Bronchoscopic Implantation Method
At the 74th Annual Scientific Assembly of the American College of Chest Physicians in Philadelphia, PA, in 2008, I had the opportunity to interview nine interventional pulmonologists with experience placing fiducial markers about the methods they use to place fiducials bronchoscopically; more than nine different methods were described during the course of the interviews. Since there are currently no commercially available delivery devices for the bronchoscopic implantation of fiducials in the lung, each of these interventional pulmonologists was forced to invent their own technique for implantation using “off-the-shelf” equipment. This is one of the main frustrations when implanting currently available fiducials and should be addressed in future lung markers.
In the following method descriptions, because there is a need to place a given fiducial inside a second device that was not designed for fiducial deployment and has not been tested for compatibility with the fiducials, the descriptions will contain more equipment specifics than are typical in these sorts of discussions. The literature and the authors experience support the compatibility of the specific fiducial/device combinations described, although other combinations are likely possible.
Additionally, all the following procedures assume that the patient has been properly consented, positioned, monitored, sedated, and anesthetized and, if fiducial implantation is part of a diagnostic bronchoscopy, that the other aspects of the bronchoscopic procedure have been completed safely, satisfactorily, and in accordance with best practice.
Mediastinal and Central Implantation Using Endobronchial Ultrasound
Implantation directly into mediastinal lymph nodes, mediastinal tumors, and central tumors using endobronchial ultrasound guidance is one of the easiest techniques to perform and therefore serves as the logical starting point. The basic technique is very similar to endobronchial ultrasound-guided transbronchial needle aspiration (TBNA).
The standard 22-gauge TBNA needle (NA-201SX-4022, Olympus America, Inc., Center Valley, PA, USA) has a removable stylet and can be used for implantation of 0.35-mm-diameter fiducials (Visicoil™, Core Oncology, Santa Barbara, CA, USA). To ensure that the fiducial is not deployed accidently, the stylet should be removed from the needle until the needle is in position within the target. The desired fiducial length for this technique is 5.0 mm to 10 mm. If the available fiducial is longer than this, it should be cut to length. To load the fiducials, the 22-gauge needle is then advanced beyond its protective catheter and the fiducial is inserted into the bore of the needle and sealed in place with sterile bone wax (Fig. 39.5).