R
Respiratory acidosis
An acid-base disturbance characterized by reduced alveolar ventilation and manifested by hypercapnia (partial pressure of arterial carbon dioxide [PaCO2] greater than 45 mm Hg), respiratory acidosis can be acute (due to a sudden failure in ventilation) or chronic (as in long-term pulmonary disease). The prognosis depends on the severity of the underlying disturbance as well as the patient’s general clinical condition.
CAUSES AND INCIDENCE
Use of certain drugs—including opioids, anesthetics, hypnotics, sedatives, and some of the new designer drugs such as Ecstasy—can be a predisposing factor for respiratory acidosis because these drugs decrease the sensitivity of the respiratory center. Other predisposing factors include central nervous system (CNS) trauma because medullary injury may impair ventilatory drive as well as ventilation therapy because the use of high-flow oxygen in chronic respiratory disorders suppresses the patient’s hypoxic drive to breathe. Neuromuscular diseases, such as myasthenia gravis, Guillain-Barré syndrome, and poliomyelitis, can result in respiratory acidosis when the respiratory muscles fail to respond properly to the respiratory drive, decreasing alveolar ventilation.
In addition, respiratory acidosis can result from chronic obstructive pulmonary disease (COPD), asthma, severe acute respiratory distress syndrome, chronic bronchitis, a large pneumothorax; extensive pneumonia, pulmonary edema, and airway obstruction or parenchymal lung disease, which interfere with alveolar ventilation.
A series of reactions leads to in respiratory acidosis. When pulmonary ventilation decreases, PaCO2 increases, and the CO2 level rises in all tissues and fluids, including the medulla and cerebrospinal fluid. Retained CO2 combines with water to form carbonic acid. The carbonic acid dissociates to release free hydrogen and bicarbonate (HCO3−) ions. Increased PaCO2 and free hydrogen ions stimulate the medulla to increase respiratory drive and expel CO2.
As pH falls, 2, 3-diphosphoglycerate accumulates in red blood cells, where it alters hemoglobin, causing it to release oxygen. This reduced hemoglobin, which is strongly alkaline, picks up hydrogen ions and CO2 and removes them from the serum.
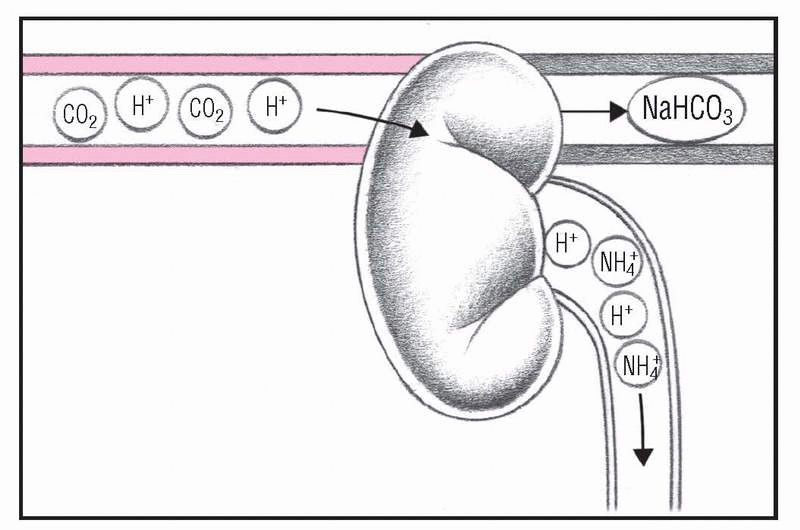
As respiratory mechanisms fail, rising PaCO2 stimulates the kidneys to retain HCO3− and sodium ions and excrete hydrogen ions. As a result, more sodium bicarbonate is available to buffer free hydrogen ions. Some hydrogen is excreted in the form of ammonium ions, neutralizing ammonia, which is an important CNS toxin.
WHAT HAPPENS IN RESPIRATORY ACIDOSIS
These illustrations explain the basic pathophysiology of respiratory acidosis.
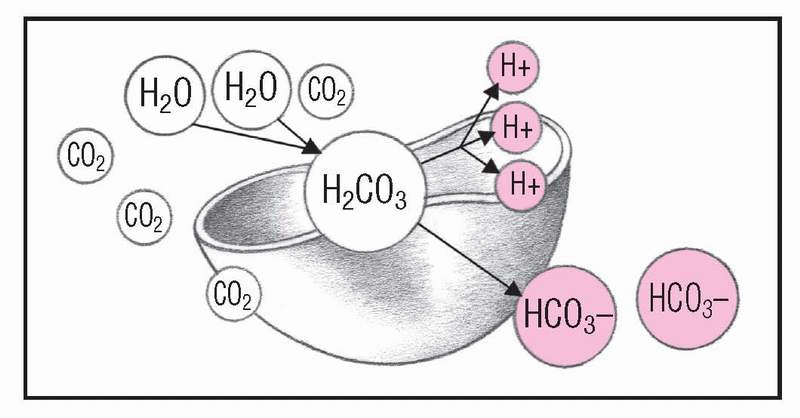
1. Pulmonary ventilation diminishes
When pulmonary ventilation decreases, retained carbon dioxide (CO2) in the red blood cells combines with water (H2O) to form excess carbonic acid (H2CO3). The H2CO3 dissociates to release free hydrogen (H+) and bicarbonate ions (HCO3−). In this condition, arterial blood gas (ABG) studies show increased Paco2 (over 45 mm Hg) and reduced blood pH (below 7.35).
|
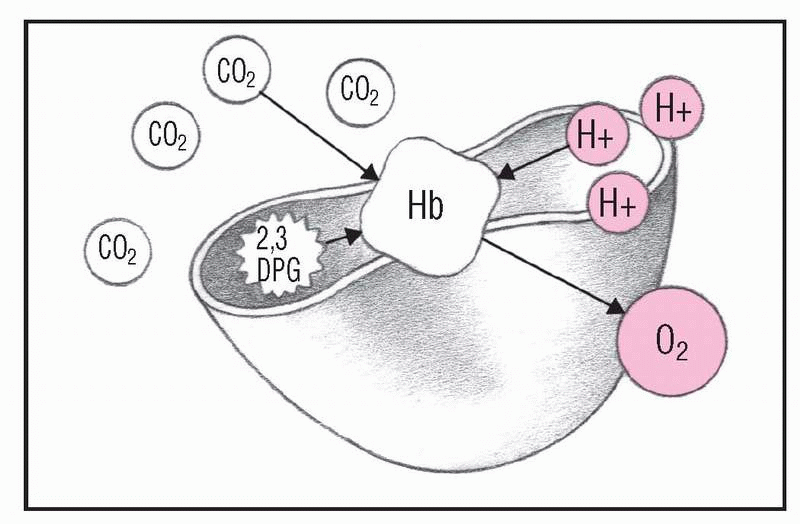
2. Oxygen saturation decreases
As pH decreases and 2,3-diphosphoglycerate (2,3-DPG) increases in red blood cells, 2,3-DPG alters hemoglobin (Hb) so it releases oxygen (O2). This reduced Hb, which is strongly basic, picks up H+ and CO2, eliminating some free H+ and excess CO2. At this stage, arterial oxygen saturation (Sao2) levels decrease, and the Hb dissociation curve shifts to the right.
|
3. Respiratory rate rises
Whenever Paco2 increases, CO2 levels increase in all tissues and fluids, including the medulla and cerebrospinal fluid. CO2 reacts with H2O to form H2CO3, which dissociates into H+ and HCO3−. Elevated Paco2 and H+ have a potent stimulatory effect on the medulla, increasing respirations to blow off CO2. Look for rapid, shallow respirations and diminishing PaCO2 levels.
|
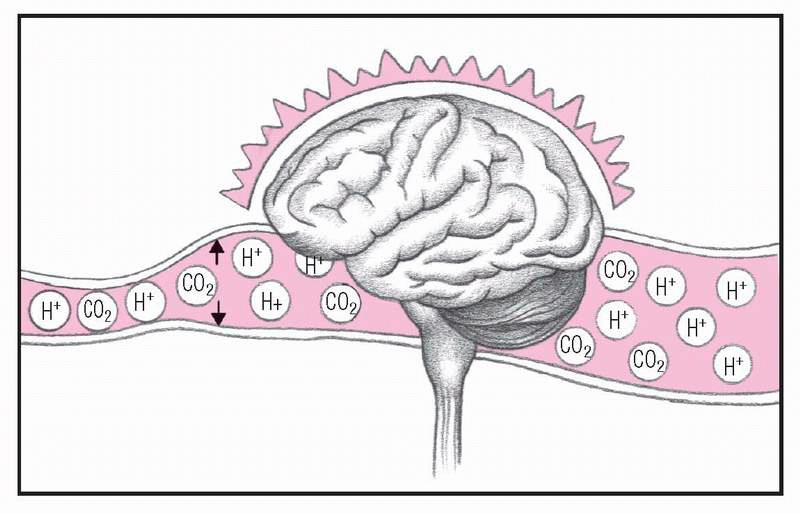
4. Blood flows to brain
The free H+ and excess CO2 dilate cerebral vessels and increase blood flow to the brain, causing cerebral edema and depressed central nervous system activity. At this stage, the patient experiences headache, confusion, lethargy, nausea, and vomiting.
|
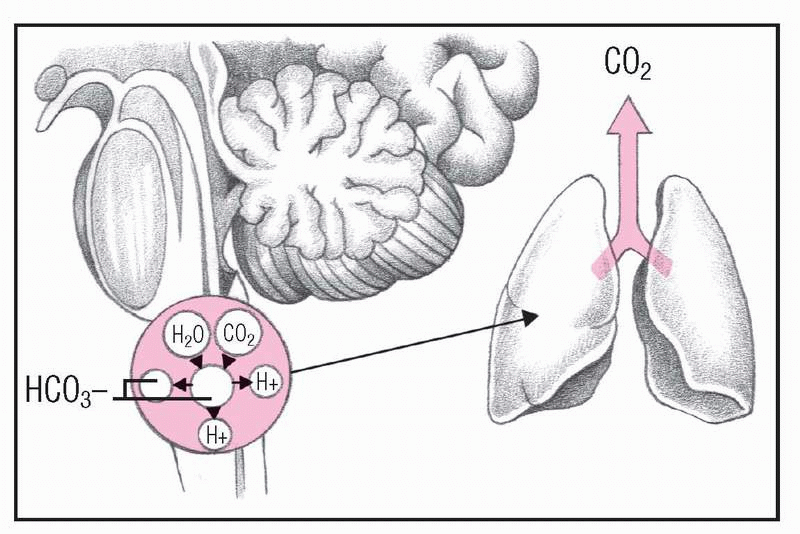
5. Kidneys compensate
As respiratory mechanisms fail, increasing PaCO2 stimulates the kidneys to retain HCO3− and sodium ions (Na+) and to excrete H+. As a result, more sodium bicarbonate (NaHCO3) is available to buffer free H+. Ammonium ions (NH4+) are also excreted to remove H+. A patient in this condition has increased urine acidity and ammonium levels, elevated serum pH and HCO3+ levels, and shallow, depressed respirations.
|
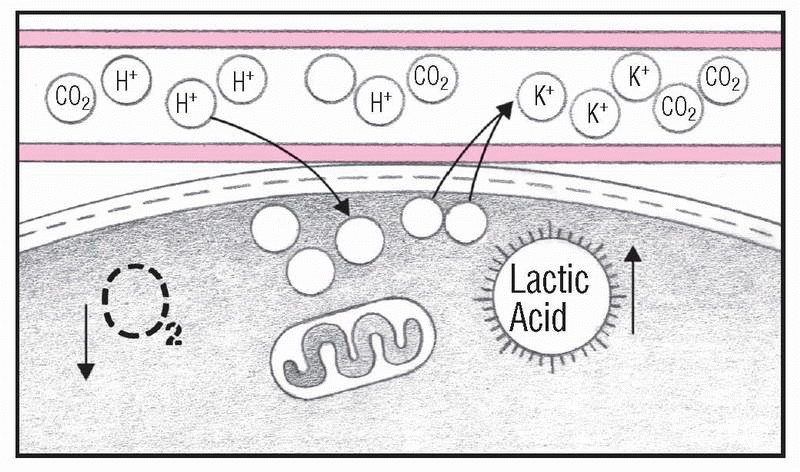
6. Acid-base balance fails
As H+ concentration overwhelms compensatory mechanisms, H+ ions move into the cells and potassium ions (K+) move out. Without sufficient O2, anaerobic metabolism produces lactic acid. Electrolyte imbalance and acidosis critically depress brain and cardiac function. ABG values in a patient in this condition show elevated Paco2 and decreased Pao2 and pH levels. The patient will experience hyperkalemia, arrhythmias, tremors, decreased level of consciousness and, possibly, coma.
|
As the hydrogen ion concentration overwhelms compensatory mechanisms, hydrogen ions move into the cells and potassium ions move out. Without enough oxygen, anaerobic metabolism produces lactic acid. Electrolyte imbalances and acidosis critically depress neurologic and cardiac functions. (See What happens in respiratory acidosis.)
SIGNS AND SYMPTOMS
Acute respiratory acidosis produces CNS disturbances that reflect changes in the pH of cerebrospinal fluid rather than increased CO2 levels in cerebral circulation. Effects range from restlessness, confusion, and apprehension to somnolence, or coma and include:
• headaches resulting from cerebral vasodilation
• fine or flapping tremor (asterixis) caused by continued elevation of CO2 levels
• dyspnea and tachypnea
• papilledema caused by increased intracranial pressure as a result of cerebral vasodilation
• depressed reflexes related to elevated CO2 levels and CNS depression
• hypoxemia
• cardiovascular abnormalities, such as tachycardia, hypertension, and atrial and ventricular arrhythmias
• hypotension with vasodilation (bounding pulses and warm periphery).
COMPLICATIONS
• Myocardial depression leading to shock and cardiac arrest
• Profound CNS and cardiovascular deterioration due to dangerously low blood pH (less than 7.15)
• Elevated PaCO2 despite optimal treatment (in chronic lung disease)
DIAGNOSIS
• Arterial blood gas (ABG) analysis confirms the diagnosis: PaCO2 exceeds the normal 45 mm Hg; pH is below the normal range of 7.35 to 7.45 unless compensation has occurred; and HCO3− is normal in the acute stage but elevated in the chronic stage.
• Chest X-rays, computed tomography scans, and pulmonary function tests can help determine the cause.
TREATMENT
Effective treatment of respiratory acidosis requires correction of the underlying source of alveolar hypoventilation. Significantly reduced alveolar ventilation may require mechanical ventilation until the underlying condition can be treated. In COPD, this includes bronchodilators, oxygen, corticosteroids, and antibiotics for infectious conditions; drug therapy for conditions such as myasthenia gravis; removal of foreign bodies from the airway; antibiotics for pneumonia; dialysis or charcoal to remove toxic drugs; and correcting metabolic alkalosis.
Dangerously low blood pH (less than 7.15) can produce profound CNS and cardiovascular deterioration; careful administration of I.V. sodium bicarbonate may be required. In chronic lung disease, elevated CO2 may persist despite optimal treatment.
Drugs
• Bronchodilators, such as albuterol (Proventil, Ventolin), metaproterenol (Alupent, Metaprel), ipratropium (Atrovent), theophylline (Theo-24, Theolair, Theo-Dur, Slobid), or tiotropium (Spiriva), to increase ventilation by decreasing muscle tone in both small and large airways in the lungs
• Benzodiazepine antagonists such as flumazenil (Romazicon) to reverse the CNS-depressant effects in patients with benzodiazepine overdose
• Opioid antagonists such as naloxone to reverse the effects of opiates and improve ventilation
Opioid antagonists may precipitate withdrawal symptoms in patients who are addicted to opiates. Also, the immediate effect is short lived and the patient needs repeated doses for the drug to continue to be effective.
SPECIAL CONSIDERATIONS
• Be alert for critical changes in the patient’s respiratory, CNS, and
cardiovascular functions. Report such changes as well as any variations in ABG values or electrolyte status immediately. Also, maintain adequate hydration.
cardiovascular functions. Report such changes as well as any variations in ABG values or electrolyte status immediately. Also, maintain adequate hydration.
• Maintain a patent airway and provide adequate humidification if acidosis requires mechanical ventilation. Perform tracheal suctioning regularly and vigorous chest physiotherapy if ordered. Continuously monitor ventilator settings and respiratory status.
• To prevent respiratory acidosis, closely monitor patients with COPD and chronic CO2 retention for signs of acidosis. Also, administer oxygen at low flow rates; closely monitor all patients who receive opioids and sedatives. Instruct patients who have received general anesthesia to turn, cough, and perform deep-breathing exercises frequently to prevent the onset of respiratory acidosis.
Respiratory alkalosis
Respiratory alkalosis is an acid-base disturbance characterized by a decrease in the partial pressure of arterial carbon dioxide (PaCO2) to less than 35 mm Hg, which is due to alveolar hyperventilation. Uncomplicated respiratory alkalosis leads to a decrease in hydrogen ion concentration, which results in elevated blood pH. Hypocapnia occurs when the elimination of carbon dioxide (CO2) by the lungs exceeds the production of CO2 at the cellular level.
CAUSES AND INCIDENCE
Causes of respiratory alkalosis fall into two categories: pulmonary and nonpulmonary. Pulmonary causes include severe hypoxemia, pneumonia, interstitial lung disease, pulmonary vascular disease, and acute asthma. Nonpulmonary causes include anxiety, fever, aspirin toxicity, metabolic acidosis, central nervous system (CNS) disease (inflammation or tumor), sepsis, hepatic failure, and pregnancy.
Respiratory alkalosis results when pulmonary ventilation increases more than needed to maintain normal CO2 levels, leading to the exhalation of excessive amounts of CO2. The consequent hypocapnia leads to a chemical reduction of carbonic acid, excretion of hydrogen and bicarbonate (HCO3−) ions, and a rising pH. In defense against the increasing serum pH, the hydrogenpotassium buffer system pulls hydrogen ions out of the cells and into the blood in exchange for potassium ions. The hydrogen ions entering the blood combine with available HCO3− ions to form carbonic acid, and the pH falls.
Hypocapnia stimulates the carotid and aortic bodies as well as the medulla, increasing the heart rate (which hypokalemia can further aggravate) but not the blood pressure. At the same time, hypocapnia causes cerebral vasoconstriction and decreased cerebral blood flow. It also overexcites the medulla, pons, and other parts of the autonomic nervous system. When hypocapnia lasts more than 6 hours, the kidneys secrete more HCO3− and less hydrogen. Full renal adaptation to respiratory alkalosis requires normal volume status and renal function, and it may take several days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree