1. Structure
(a) Facility
(b) Equipment
(c) Staff
2. Process
(a) Patient selection
(b) Study performance
(c) Study interpretation
(d) Study reporting
3. Outcome
(a) Death
(b) Disease
(c) Discomfort
(d) Disability
(e) Dissatisfaction
Table 14.2
Quality initiatives in pediatric and congenital echocardiography
1. Accreditation |
(a) Sonographers |
(b) Physicians |
(c) Echocardiography laboratories |
2. Productivity standards |
3. Appropriate use criteria |
4. Normal reference values |
5. Quality metrics |
(a) Reporting for critical results |
(b) Adverse events with sedated echocardiograms |
(c) Variability in pediatric echocardiographic measurements |
(d) Diagnostic errors |
(e) Study completion |
(f) Image quality |
Structural Indicators
Structural indicators refer to the type and amount of resources needed to provide quality echo services [3], specifically in terms of the facility, the equipment, and the staff.
Facility
Determinants of quality services within the facility include the physical space, availability of patient privacy, patient volume, and daily workflow. How big is the echo lab? How many examination rooms are available? Does the physical space account for patient privacy? How much time is allotted for each study? How many patients can the facility accommodate at any time? How many patients are evaluated in the echo lab every year? What happens when studies are being performed? How is supervision provided to sonographers and fellows within the facility’s physical space and daily workflow? Where and how are the studies reviewed?
Equipment
Equipment maintenance, software, and safety practices certainly play important roles in the provision of quality services. How many echo machines does the facility have? Are the machines old or new? Is all the necessary software for the machines and for the digital echo system updated to the most recent version? Are all the machines and other equipment compliant with standards from The Joint Commission? Are they compliant with IAC standards?
Staff
Lastly, staffing indicators involve individual responsibilities and workload in the context of established benchmarks. Are the clinical and administrative responsibilities clearly delineated within the echo lab? Who manages the workflow? Who provides supervision? Are there system checks to assure that workload is not excessive and not associated with increased errors? Does the echo lab meet established benchmarks in terms of staffing responsibilities and workload?
Process Indicators
Process indicators involve the activities and tasks needed to provide quality echo services [3]. The components of an echo that serve as indexes of quality include patient selection, study performance, study interpretation, and study reporting [1].
Patient Selection
Quality assessment as it pertains to patient selection necessarily involves the reported indication and its appropriateness. In 2007, the ACC Foundation, ASE, and five other subspecialty societies published a report on appropriate use criteria (AUC) for transthoracic and transesophageal echo studies in adults organized by indication categories and based on specific methodology developed by the RAND Corporation and researchers from the University of California, Los Angeles [4]. Only 4 years later, the same group along with three additional organizations drafted a revised AUC document to include criteria for stress echo, to account for changes in clinical practice and test utilization patterns, and to address deficiencies in the original publication [5]. Combining evidence-based medicine and practice experience data during the vetting process, the new document lists over 20 categories and almost 100 indications for transthoracic echo, each one categorized as appropriate, inappropriate, or uncertain criteria, and it has become an important component of the IAC guidelines and standards for adult echo. A similar document does not exist for pediatric echo, mostly because of the potential for a significantly extensive list of indications, especially if the criteria need to be specific for each CHD. Interestingly, the 2011 document lists 7 indications for adult CHD with only 4 being labeled as appropriate.
Study Performance
Another important indicator involves study performance, which is determined primarily by established guidelines and standards, individual lab protocols, and sonographer knowledge. Publications outlining recommendations based on expert consensus certainly provide a framework in which to assess study performance quality. Over the last 4 years, the ASE has published over 15 guidelines documents encompassing such topics as diastolic function [6], prosthetic valves [7], right heart evaluation [8], cardiac mechanics [9], and 3-dimensional echo [10], but all of these documents pertain only to studies performed in adults. Nevertheless, the ASE has published 5 pediatric documents since 2004 establishing recommendations pertaining to the performance of a pediatric echo [11], quantification methods during a pediatric echo [12], performance of a pediatric transesophageal echo [13], performance of a fetal echo [14], and targeted studies in the Neonatal Intensive Care Unit [15].
Study performance within an echo lab is dependent on protocols that are based on published guidelines and standards, but the specific contents of the protocols must be established in the context of local culture, clinical practice, and experience. Many centers have developed lesion-specific echo protocols in an effort to prevent sonographers from forgetting the most important aspect of each lesion during the performance of a study, definitely contributing to quality echo services.
General and local algorithms for study performance enhance sonographer knowledge, but this knowledge also depends on experience and education. It definitely requires experience to know when non-standard views are needed to evaluate specific lesions, such as complete atrioventricular canal defects or tetralogy of Fallot (Fig. 14.1). From an education standpoint, all sonographers must attend ultrasound school, but only a few schools have established a specific pediatric or congenital curriculum. Hence most sonographers begin working in a pediatric echo lab with minimal knowledge and experience, and their improvement is usually dependent on learning from senior sonographers and physicians. In addition, the infrastructure must ensure continuing education with regular didactic sessions at the center as well as at local, regional, and national conferences.
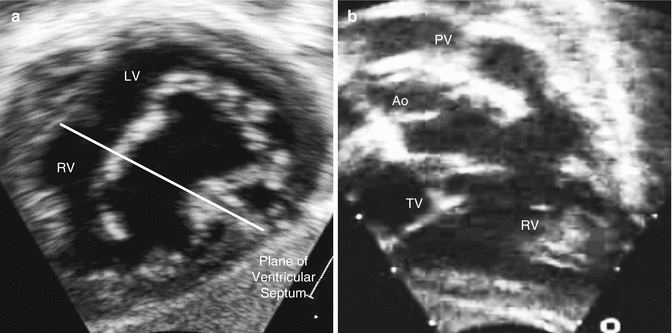

Fig. 14.1
Non-standard echocardiographic views for specific lesions: (a) “en face” or “in-between” view in subcostal windows of a complete atrioventricular canal depicting a cross-section en face view of the common atrioventricular valve, usually obtained at a plane between the long-axis and short-axis subcostal view; (b) right axial oblique view in subcostal windows of tetralogy of Fallot depicting the right ventricular inflow and outflow as well as the anterior deviation of the conal septum, usually obtained with counterclockwise rotation of the probe from the standard long-axis subcostal view (Ao aorta, LV left ventricle, PV pulmonary valve, RV right ventricle, TV tricuspid valve)
Study Interpretation
Physician knowledge is the most crucial determinant of quality interpretation of studies. As with sonographer knowledge, this is dependent on education and experience. The Accreditation Council for Graduate Medical Education (ACGME) is fairly specific in terms of the body of knowledge needed during the training of pediatric cardiology fellows [16]. In addition, the ACC Foundation, AHA, and American Academy of Pediatrics (AAP) have established training guidelines for pediatric noninvasive cardiac imaging with very specific goals for core and advanced imaging skills [17]. In fact, many of the major clinical centers in North America have developed 4th year advanced imaging fellowships for trainees who want to focus specifically on echo or some other aspects of noninvasive imaging.
The role of experience in developing physician knowledge cannot be stressed enough. Experience allows one to understand how variants of abnormal physiology can have different effects on abnormal morphology, such as the presentation of subaortic stenosis as a solitary lesion or in association with a ventricular septal defect and an interrupted aortic arch (Fig. 14.2). Experience is crucial in the diagnosis of rare anomalies such as a persistent 5th arch or a retro-aortic innominate vein (Fig. 14.3). In fact, the ACGME as well as the IAC and other regulatory bodies are quite specific in terms of the target number of studies for trainees prior to graduating from a fellowship program and for echo physicians as part of the echo lab accreditation process [1, 16, 18].
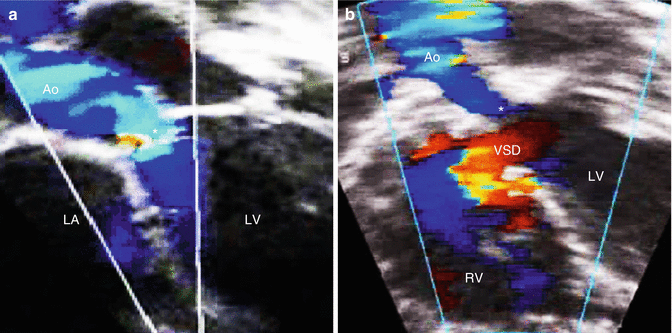
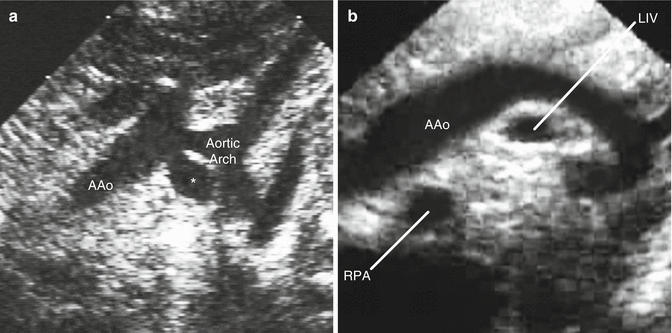

Fig. 14.2
Variable echocardiographic presentation of subvalvar aortic stenosis based on the pathophysiology and associated anomalies: (a) isolated subvalvar aortic stenosis (star) revealing turbulence in flow along the area of obstruction; (b) subvalvar aortic stenosis (star) in association with a ventricular septal defect, an interrupted aortic arch, and a large patent ductus arteriosus (the last two lesions are not shown) revealing absence of turbulence along the subaortic region because of left-to-right flow at the ventricular septal defect and right-to-left flow at the patent ductus arteriosus; in this instance, there is still significant obstruction along the subaortic region despite the absence of turbulence because of the pathophysiology of the associated lesions (Ao aorta, LA left atrium, LV left ventricle, RV right ventricle, VSD ventricular septal defect)

Fig. 14.3
Rare anomalies: (a) persistent 5th aortic arch (star) revealing two aortic arches coursing from right to left (in contrast with a double aortic arch where one arch courses to the right and the other to the left); (b) retro-aortic innominate vein which courses behind the ascending aorta and is seen as two vessels in cross-section below the aortic arch in suprasternal long-axis views (the second vessel is the right pulmonary artery) (AAo ascending aorta, LIV left innominate vein, RPA right pulmonary artery)
The quality of study interpretation is also determined by validation activities within an echo lab to evaluate the accuracy and repeatability of echo findings and measurements. Many reports have been published comparing echo diagnoses with data from other modalities (such as cardiac magnetic resonance imaging, computed tomography imaging, and cardiac catheterization) as well as findings in the operating room during surgery [19–22]. More recently, other publications have evaluated multi-acquisition, intra-observer, and inter-observer variability for echo measurements and have shown that some measurements are more prone to poor repeatability than others, even with multiple heart beat averaging [23]. The IAC has developed specific recommendations for quarterly correlation assessments (to compare echo findings in specific instances with results from other modalities and from the operating room) and variability assessments (to evaluate the repeatability of specific measurements performed during an echo) [18].
Study Reporting
The reporting of echo findings has certainly been fraught with problems, particularly in terms of the inconsistent terminology and coding systems for CHD. Controversies in nomenclature originate partly from the different approaches used to describe the morphology of cardiac malformations and their variants, such as seen with heterotaxy syndrome [24]. In the current era of digital echo, individual diagnoses and procedures are coded within the structured reporting platform of each system, and the organization of codes is usually customizable and frequently based on consumer preference. Therefore, although databases of echo diagnoses have been established at individual institutions, the ability to share data between centers can be quite limited, especially if each center uses a different digital echo vendor and creates its own customized structured reporting platform. The varied and heterogeneous coding systems that are currently in use include multiple American and European codes created by pediatric cardiology or cardiac surgical organizations, codes from the 9th, 10th, or 11th revision of the International Classification of Diseases (ICD-9, ICD-10, or ICD-11) developed by the World Health Organization, and the Current Procedural Terminology (CPT) codes established by the American Medical Association. Efforts by the ISNPCHD to cross-match these codes have resulted in the International Pediatric and Congenital Cardiac Code, which now provides a framework in which to establish a common diagnostic and procedural coding system [25, 26].
Another issue relevant to quality reporting involves the standardization of reports within an institution, usually involving a description of relevant positive and negative findings organized in a segmental fashion, a list of all measurements along with reference values based on the patient’s age and body size, a summary of the patient’s clinical history, and a hierarchical summary of the important findings during the evaluation. In addition, local standards must be established in terms of turnaround time (time from study completion to report completion), reporting of critical findings to relevant health care providers, automatic distribution of reports to appropriate physicians and inpatient locations within the institution, and their incorporation into the medical records. Again the IAC has developed specific recommendations and guidelines for study reporting that are important components of the accreditation process for an echo lab [18]. Lastly, reports should provide recommendations if other modalities are needed to establish or confirm a diagnosis, thereby functioning as the 1st step in deciding what interventions if any are needed for a specific patient.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


