Fig. 5.1
Typical quality control steps
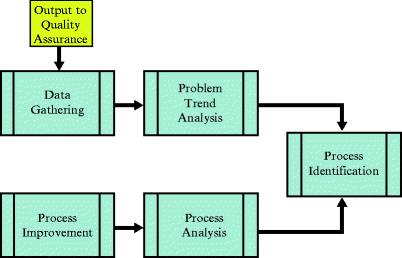
Fig. 5.2
Typical quality assurance steps
Methods of Quality Improvement
Developing a quality monitoring process requires the use of an established system. This system can be as simple as those outlined in Figs. 5.1 and 5.2 to processes that require advanced instruction. There exist numerous monitoring methods to facilitate consistency and standardization in performing monitoring. Next, several well-established methods of quality improvement will be discussed. Many of these systems have similarities, but the overall implementation must be user friendly to the team using these tools.
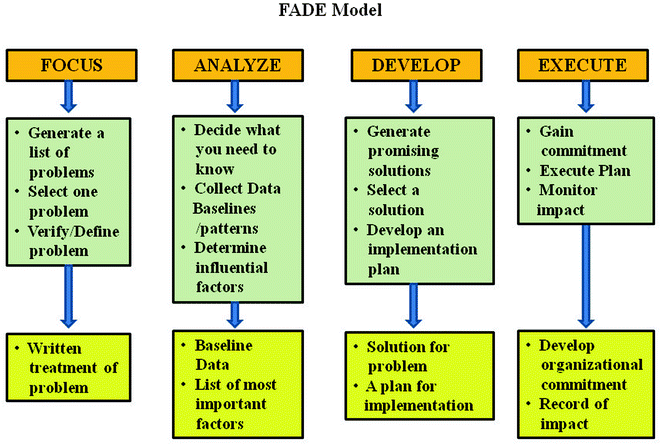
The FADE Model
The FADE model was developed by Organizational Dynamic Institute of Wakefield, Massachusetts. There are five steps in using this model for a quality improvement project (Fig. 5.3):
1.
Focus: A process or problem to be improved must be clearly defined at the outset.
2.
Analyze: Data must collected and analyzed to establish a baseline. From this analysis, the goal is to identify the root causes of the problem being studied.
3.
Develop: Based upon the data analysis, possible solutions are evaluated and an action plan for improvement is created. This should include components of implementation, communication, and the system of measuring and monitoring to be used after the program has been put in place.
4.
Execute: The process that has been developed is now put into effect. If this is a large-scale program, for instance, at an institutional level, pilot programs should be considered to test the process and monitoring systems developed.
5.
Evaluate: It is also at this step that reevaluation of the original goals of the project is reviewed and any modifications of the program developed or variables measured are to be corrected. Once the process of improvement has begun, an ongoing monitoring process must remain in place to maximize the effects of the quality insurance program instituted and ensure its long-term success.

Fig. 5.3
FADE
PDSA
Another commonly used quality improvement technique is the PDSA cycle:
1.
Plan: An initial idea or issue for change must be formally identified. Contributions from members of the team involved or a quality committee can be used to fully define the proposal.
2.
Do: The plans that develop in number 1 must be carried out according to the parameters set.
3.
Study: The results that are created by implementing the plan are then evaluated.
4.
Act: After the results have been adequately analyzed, a plan of action must be developed to improve the quality program developed.
After the action has taken place, a new plan is formulated, and the process is repeated until the desired goal is achieved.
Six Sigma
Six sigma is a much more comprehensive program than can be effectively taught by a single chapter. Six sigma originates from a statistical modeling technique for manufacturing processes. A six sigma process is one in which 00.00066% of the products manufactured are expected to be free of defects (3.4 defects per million units). Six sigma is a name coined by Motorola, USA, in 1986, to identify a business management strategy they had developed. Six sigma has evolved over time as a method to improve the quality of process outputs by identifying and removing the causes of defects or errors in a process. Six sigma techniques also help to minimize variability in manufacturing, business, or other processes. Much of the new LEAN techniques of quality control being taught at health care systems is based on the six sigma philosophy.
The two major techniques used as part of six sigma:
1.
DMAIC: This is used for projects designed to improve existing processes. This project methodology has five phases:
(a)
Define the problem.
(b)
Measure and collect relevant data.
(c)
Analyze the data collected and identify a cause-and-effect relationship.
(d)
Improve the current process.
(e)
Control the process to ensure that any deviations from the new targets are identified quickly. This step also requires implementing systems of ongoing measurement.
2.
DMADV or DFSS: These systems are used for projects developed to create new products or processes, such as adding a new procedure to the bronchoscopy section.
(a)
Define the problem.
(b)
Measure and collect relevant data.
(c)
Analyze the data collected and identify a cause-and-effect relationship.
(d)
Design the details needed for the new product/process. In more advanced systems, this could require simulations or modeling.
(e)
Verify that the design will work. Plan and execute a pilot run to identify any potential mistakes in the process.
Other
The goal of quality improvement can be attained using less sophisticated tools for analysis than the FADE, PDSA cycle, or Six Sigma:
1.
Flow charting process: Flow charting can be used to assess a process within the bronchoscopy suite itself. This technique can identify process issues at any level throughout patient or instrument throughput. Bottlenecks of flow are usually identified, allowing program improvement with correction of these areas of concern.
2.
Self-reporting of critical incidents: In many ways, this is what morbidity and mortality evaluations should be. If the review of these events does not involve identification of system or personnel problems, it has not been effective as a quality improvement tool.
3.
Database development: Using a database that prospectively collects information allows evaluation of problems which may arise, in a more dynamic fashion that having to return to retrospective data review on every occasion. Databases can be used at institutional levels, system levels, or nationally as is seen with large registries (i.e., patient registry for primary pulmonary hypertension, Pennsylvania Idiopathic Pulmonary Fibrosis State Registry, etc.).
Quality Control Measures for Endoscopic Procedures
Areas of potential quality control intervention have been divided into:
1.
Procedural processes
2.
Equipment use in procedures
3.
Other
These topics are only a basic outline for potential areas of quality control projects. The examples given are exactly that examples given to help illustrate problems encountered with solutions.
Procedural Processes
Procedural processes can be influenced by the practice at individual institutions. If bronchoscopy is performed in a bronchoscopy suite vs. an endoscopy area vs. an operating room, there are different administrative and leadership issues, which can affect the overall procedural processes of bronchoscopy. Procedural processes include the standardized performance of procedures, patient management protocols, complication rates, equipment failures, diagnostic yields, and registries.
A. Performing Procedures
At most institutions, there are multiple bronchoscopists working with multiple fellows, nurses, respiratory therapists, and other personnel in the performance of bronchoscopies. If every combination of personnel is using individual preferences in the performance of their portion of the bronchoscopy, there would be very poor efficiency for the entire team. Efficiencies can affect not only the yields of procedures but the time needed to perform the procedure, the time necessary to set up or break down the room, risk the proper processing of the specimens, and increase the risk to patients as well as equipment.
Performing procedures can be broken down into functional components that quality control issues can more specifically address.
1.
Procedural Setup
(a)
Focus: To standardize the setup of the therapeutic table in the operating room. Multiple nurses and technicians were occasionally required to set up the room, particularly on off hour cases. Many instances of not having the correct equipment on the table or not being able to identify the location of some equipment on the table occurred.
(b)
Analyze: Notes were made on the various setups and physician variances.
(c)
Develop: All physicians, nurses, and technicians agreed on what equipment was necessary for the table and how it would be positioned.
(d)
Execute: Pictures of the “accepted” table setup were taken. Inservices were completed for all personnel. Photos are left in the operating room for any personnel to refresh their memory about room requirements.
(e)
Evaluate: The program still exists. The photos remain in the operating room easily available for review, and inservices are held for all new personnel on a biyearly basis. The setup time has been reduced by the repetition of setting up the same table. The process had additionally selected all ancillary supplies to be used with each case. These supplies are now stored in the operating room to make them easily available for initial setup and/or replacement during the procedure, and during procedures, all physicians and supporting staff have been informed where each piece of equipment is located (Fig. 5.4).
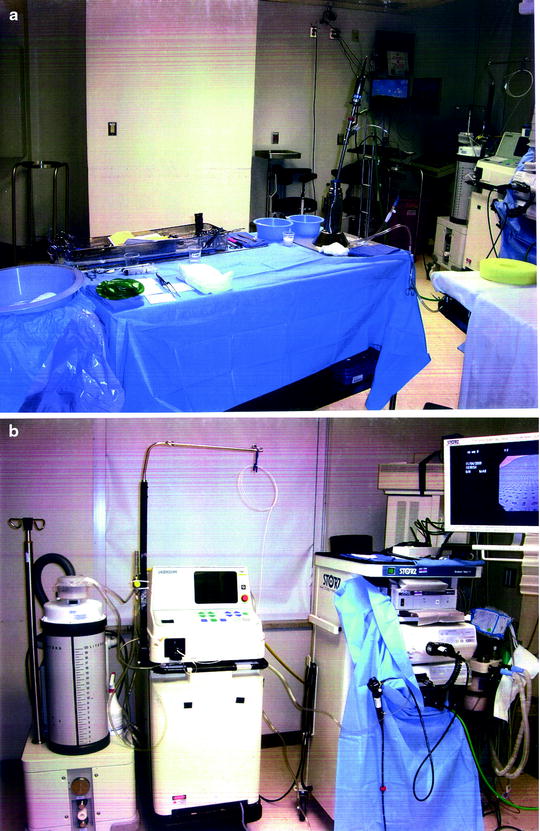
Fig. 5.4
(a) Table setup; (b) Room setup
2.
Medication monitoring
New medications and various recommendations are continuously changed and modified for the use of medications. Combinations of narcotics, benzodiazepines, hypnotics, and inhalational agents can be used for sedation during bronchoscopy. It is necessary to choose effective combinations of medications to be used for moderate, deep, or general anesthesia:
(a)
Use of medications should be standardized within the bronchoscopy suite. This will ensure expected responses and results to the medications, allowing the appropriate reversal agents to be readily available.
(b)
When the medications are chosen, appropriate compliance review data must be collected. These data sheets are collected and analyzed comparing data to medication use (Fig. 5.5).
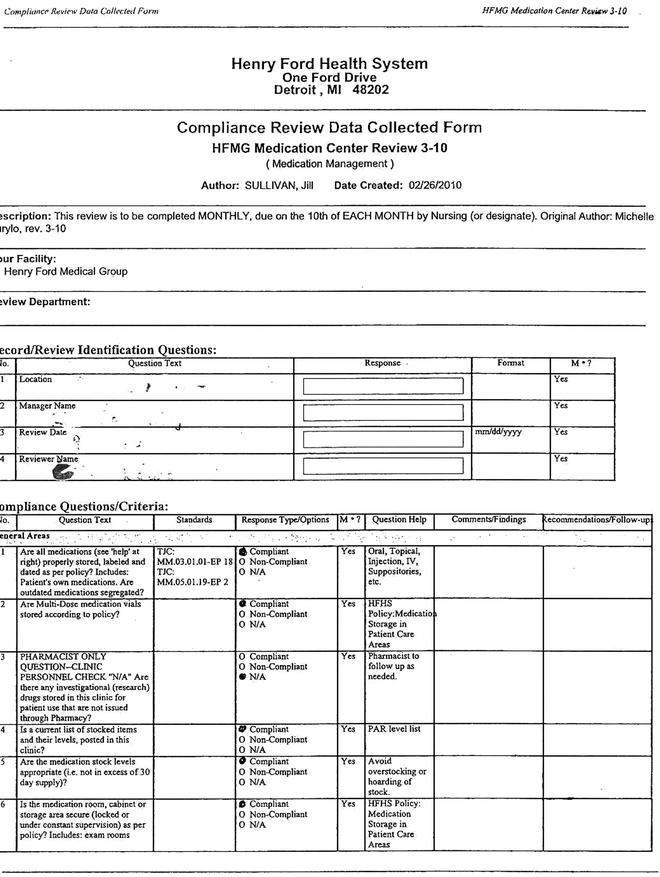
Fig. 5.5
Compliance review data collected form
3.
Procedural Standardization and Patient Management Protocols
(a)
Personnel should all be practicing using similar procedural protocols. If there are significant outliers to the performance of procedures, then any variances in morbidity, mortality, or diagnostic yield cannot be easily assessed:
i.
The number of transbronchial biopsies that should be routinely performed for the bronchoscopic assessment of a mass or solitary pulmonary nodule
ii.
The amount of fluid instilled and the amount expected on return to perform a bronchoalveolar lavage (BAL)
iii.
Appropriate mediastinal staging with EBUS TBNA following lymphatic drainage maps of lobar lymphatic flow
iv.
Get Clinical Tree app for offline access

Establishing the appropriate management protocols, for example:
1.
Complex tracheal stenosis
2.




Endobronchial carcinoid tumors treatment and follow-up
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


