Chapter 33 Pulmonary surgery
 Surgical resection of lung tissue via a thoracotomy is a routine procedure, used mostly for treating lung cancer, which requires careful assessment of the patient’s physiological reserve.
Surgical resection of lung tissue via a thoracotomy is a routine procedure, used mostly for treating lung cancer, which requires careful assessment of the patient’s physiological reserve. Less invasive surgical techniques such as video-assisted thoracic surgery are increasing rapidly and associated with less physiological disturbance and complications.
Less invasive surgical techniques such as video-assisted thoracic surgery are increasing rapidly and associated with less physiological disturbance and complications. One-lung ventilation is required for many pulmonary surgery procedures and understanding of the physiology involved is vital for its safe use.
One-lung ventilation is required for many pulmonary surgery procedures and understanding of the physiology involved is vital for its safe use. Lung transplantation is an established technique for treating advanced lung disease, with chronic obstructive pulmonary disease currently being the most common indication.
Lung transplantation is an established technique for treating advanced lung disease, with chronic obstructive pulmonary disease currently being the most common indication.Physiological Aspects of Common Interventions
Bronchoscopy
Flexible bronchoscopy.1 The flexibility of fibreoptic bronchoscopes allows a view of all the major branches of the tracheobronchial tree with minimal risk of trauma and discomfort for the patient. The procedure can therefore be performed without general anaesthesia, though extensive topical anaesthesia to the airway is required, and most clinicians also provide sedation to relieve the anxiety associated with having a bronchoscopy.2 Hypoxia during a flexible bronchoscopy is common,1 occurring in 17% of patients from one study,2 and supplemental oxygen is therefore normally used. Lung function during bronchoscopy is significantly impaired. Whilst the bronchoscope is in place the functional residual capacity (FRC) is increased by 17%,3 and forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow are all decreased,4 indicating airflow obstruction. These observations are not explained simply by the presence of the bronchoscope in the airway, as the observed airway flow limitation begins after the airway local anaesthetic is applied (before insertion of the bronchoscope) and continues for several minutes after the bronchoscope has been removed, suggesting that a bronchoconstrictor action of the topical anaesthesia is responsible.4 Respiratory depression may also occur during or soon after bronchoscopy, and the causes of this are uncertain but likely to relate either to the sedative drugs or the topical anaesthesia in the airway. The major limitation of flexible bronchoscopy is the size of the instruments that may be passed down the bronchoscope, and though very suitable for visualisation of the airway and obtaining biopsies or washings, for removal of foreign bodies or airway surgery a larger portal for access to the tracheobronchial tree is required.
Ventilation during a rigid bronchoscopy with general anaesthesia is a challenge and four main techniques may be used:5
Thoracoscopy
Thoracotomy
A surgical opening in the chest cavity was first used more than 100 years ago, usually for the treatment of empyema (page 448) and tuberculosis. In current surgical practice the indications for thoracotomy have widened to include surgery of the lungs, major vessels, oesophagus and thoracic spine. In most cases, thoracotomy is performed in the lateral position which has significant effects on respiratory physiology (see below) and through a postero-lateral incision. Thoracotomy includes division of the muscles exterior to the ribcage and entering the pleura, usually through the 5th intercostal space, by separating the intercostal muscles from the rib. The rib adjacent to the thoracotomy is commonly divided or a piece of rib resected to improve access and to minimise rib fractures when the chest wall is retracted.
The effects of thoracotomy on postoperative respiratory function are profound, with significant reductions in chest wall compliance and respiratory muscle activity11 resulting from chest wall oedema, pain, disruption of muscle anatomy, and later in the recovery phase scarring of chest wall tissues. In the first 24 hours following surgery, FVC and FEV1 are only 30–50% of the preoperative volumes, with some evidence that the type of thoracotomy incision used may affect these values.12 Chest wall compliance falls to around 60% of the preoperative value by the third postoperative day before slowly improving.13 At one week after surgery, FVC and FEV1 are around 70–80% of preoperative values, and by this stage the different incisions seem to have little effect on recovery.14,15
Other measures of respiratory muscle strength such as maximum inspiratory and expiratory mouth pressures are also reduced to about half the preoperative values following thoracotomy, and in one study had not returned to normal 12 weeks after surgery.16 The same study showed a rapid return to normal of both measures of muscle function following VATS procedures. Older patients, who have poor respiratory muscle strength relative to younger patients, took longer to recover muscle function following surgery, possibly explaining the greater incidence of pulmonary complications with increasing age. Thoracotomy alone therefore impairs respiratory muscle function to such an extent that ventilation may not be able to keep pace with the extra ventilatory requirements associated with having major surgery, and alveolar hypoventilation can occur along with regional pulmonary collapse and impaired oxygenation. Even in patients less severely affected the ability to cough is always weakened with an increased risk of chest complications.11 For patients who have a lung resection through their thoracotomy, lung compliance is also decreased to about half their preoperative value, compounding the above problems.13
Considering the surgical trauma associated with thoracotomy, it is unsurprising that there is commonly severe pain in the postoperative period. Damage to somatic nerves supplying the skin and chest wall structures is exacerbated by trauma to the visceral nerves supplying the pleura and possibly by involvement of the sympathetic chain in the chest cavity. Because all three of these nerve pathways may be involved, treatment of acute postoperative pain is challenging, with multimodal treatment regimes being required. However, of more significance to the patient is the observation that following a thoracotomy almost half of patients develop a chronic pain syndrome, the pathophysiology of which remains unknown, though genetic and psychological factors are believed to be important contributors.17
Lung Resection
Assessing patient fitness for lung resection.18,19 Lung function is assessed using either the FEV1, or, if the patient has parenchymal lung disease, the diffusing capacity for carbon monoxide (Dlco, page 153). If these are less than 80% of normal predicted values for that patient, an attempt is made to calculate predicted postoperative values based on which anatomical sections of lung need to be removed. Radionucleotide ventilation or perfusion scans or quantitative computerised tomography scans may all be used to measure functional lung units, and are useful techniques as they also show which pathological lung units are already not contributing to overall function. Less invasive is the anatomical method in which the lungs are divided into 19 anatomical segments of equal value, and knowing which segments are to be removed enables estimation of postoperative predicted lung function. For many years a general rule of lung resection was that a predicted postoperative FEV1 of less than 0.8–1.0 l precluded resection, though evidence for this rule is poor. Using an absolute value for FEV1 or Dlco is fraught with difficulties as sex, age and height all affect the normal values, and decisions should now always be based on the values as a percentage of the predicted normal for that patient.
Different studies have produced varied results on the association between percentage predicted postoperative FEV1 or Dlco and outcome, but a value of less than 40% of predicted normal is now generally accepted as being associated with an increased mortality and complication rate. For patients in this situation, measurement of preoperative exercise tolerance has the advantage of also including a cardiovascular component to the assessment and may help to further define risks and outcomes. The most objective way of quantifying exercise activity is by measuring Vo2max (page 262). Values of less than 15 ml.min−1.kg−1 are again associated with poor outcome. Clinical measures of exercise tolerance have some value, but these must be performed under supervision as patients’ own reported exercise tolerance is normally greatly exaggerated. Tests which have some limited use in predicting outcome after lung resection include the shuttle test (number of times a patient can walk between two markers 10 m apart), 6-minute walk test (distance able to walk within 6 minutes) and stair climbing (the number of stairs or height of stairs the patient is able to climb20).
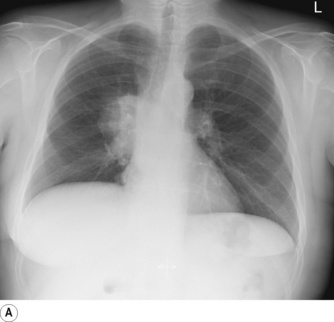
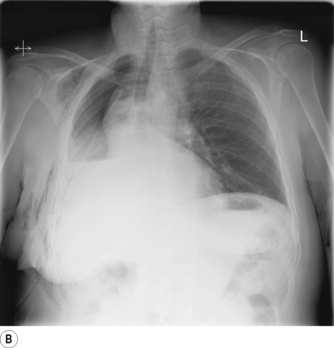
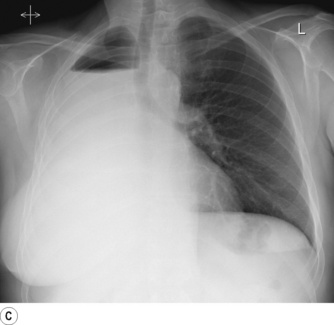
Pneumonectomy. Resection of an entire lung is usually performed for removal of large, central lung tumours (Figure 33.1A). Following pneumonectomy correct management of the empty hemithorax is crucial. If air is drained from the cavity too quickly mediastinal shift will occur which impairs venous drainage to the heart and can cause cardiovascular collapse. The most common practice is therefore to leave no drain in the chest cavity, and monitor the position of the mediastinum daily with a chest x-ray. Alternatively, a chest drain can be placed (Figure 33.1B), but be clamped for most of the time and only released briefly and intermittently to ensure the pressure in the cavity is approximately atmospheric. A more interventional approach is to measure the pressure in the chest cavity and instill or remove air to maintain a pressure of −2 to −4 cmH2O on inspiration and +2 to +4 cmH2O on expiration. Within a few weeks of pneumonectomy the volume of the hemithorax decreases due to a combination of mediastinal shift, elevation of the diaphragm, and contraction of the chest wall, and pleural fluid replaces the air in the chest cavity (Figure 33.1C). Over the ensuing months or years, the fluid volume decreases as the mediastinal shift continues, and the other lung herniates anteriorly or posteriorly across the midline to partially fill the vacated hemithorax. Complete replacement of the fluid is however unusual.
Recent studies in animals have demonstrated the intriguing phenomenon of ‘neoalveolarisation’ following lung resection.21 Within 20 days of lung resection in mice the number of alveoli in the remaining lung increased by 50%, completely restoring the gas exchanging surface area.22 Neoalveolarisation probably occurs by new alveoli forming in the walls of existing alveolar ducts and respiratory bronchioles, and is so far only described in young animals, as would be expected from the observation that in mammals formation of alveoli is a post-natal process (page 250). It therefore remains only a distant prospect that adult patients will be able to grow new lung tissue after lung resection.
Lung injury following pneumonectomy.23,24 Acute lung injury (Chapter 31) is a serious complication that occurs in the postoperative period in between 2.5% and 9% of pneumonectomies, and more rarely follows smaller lung resections such as lobectomy. Mortality is high, with a quarter of patients dying, though this is an improvement compared with only a few years ago. The pathophysiology of post-pneumonectomy acute lung injury is controversial, with perioperative fluid overload being viewed by many clinicians as the main cause, though the pathophysiology is now better elucidated and far more complex than simply administering excessive volumes of intravenous fluid. High-protein pulmonary oedema develops approximately 24 hours postoperatively, and is believed to result from endothelial cell injury in the pulmonary capillaries. How this initial injury occurs is less clear, though the increased capillary blood flow in the remaining lung is likely to cause stretching of endothelial cells or excessive shear forces in the vessels, both of which may disrupt the inter-cellular junctions. Overdistension of the lung either during surgery with inappropriately large tidal volumes25 or use of PEEP, or following surgery with sub-optimal management of the contralateral chest cavity may all contribute to further disruption of the alveolar–capillary barrier.23 Once this initial lung injury has occurred, many other factors will then affect the severity of the clinical picture and its management, including fluid administration, inspired oxygen levels and the ventilation strategy, all of which should follow the same principles as for the management of ALI whatever the cause (Chapter 31).
Surgery for Chronic Obstructive Pulmonary Disease (COPD)26
Surgical treatment is reserved for patients with severe COPD in whom emphysematous changes predominate. When the airspaces created in emphysema become larger than 1 cm in diameter they are referred to as a ‘bulla’. Nearby bullae can merge and result in extremely large air spaces, occupying up to one-third of the lung volume. Like emphysema, bullae have little effect on gas exchange as both tidal ventilation and blood flow to the bulla are negligible. However, with giant bullae the airspace acts in a similar fashion to a pneumothorax (page 445) and compresses surrounding lung tissue, causing further worsening of airway collapse and subsequent disturbance of gas exchange. In these cases surgical treatment involves ‘bullectomy’, and with careful patient selection this can be a useful operation. Improved surgical techniques led to a resurgence of interest in surgery for COPD and extended the indications to include patients who do not have bullae.
Lung volume reduction surgery (LVRS) involves removing 20–30% of lung volume, to include the most emphysematous areas, and can have impressive results. Improved long-term survival compared with best medical therapy has only been proven in patients with poor exercise capacity and upper lobe emphysema,27,28 and conversely, patients with high exercise capacity and emphysema elsewhere in the lung have a higher mortality following surgery compared with medical treatment. Despite these mixed survival results, in appropriately selected groups of patients LVRS can improve exercise capacity,27,29 lung volumes, quality of life30 and arterial Po2.31 Understanding of the physiological mechanisms leading to clinical improvements remains incomplete. Potential benefits of LVRS include reduced pulmonary collapse adjacent to emphysematous areas, improved elastic recoil of the remaining lung tissue, and better respiratory muscle function, particularly of the diaphragm,32 secondary to reduced hyperinflation (see Figure 6.1).
Pleurodesis33
Pleurodesis describes a variety of procedures, all of which aim to induce adhesions between the visceral and parietal pleura. The two most common indications are pneumothorax that has failed to respond to conservative management (page 456) or palliation of malignant pleural effusion. Though the preferred technique varies with the indication, the success of any pleurodesis depends upon inducing inflammation in the pleura whilst simultaneously ensuring the two pleural layers are closely apposed, so allowing the normal inflammation and tissue repair processes to cause scarring in the pleural space. Apposition of the pleura is usually achieved by using a pleural drain, but if required an inflammatory reaction in the pleura can be initiated by various means. A pleurectomy may be performed, with the parietal pleura being simply stripped from the inside of the chest wall, or a less traumatic technique is pleural abrasion in which the pleura is rubbed with a dry gauze or other abrasive surface. Alternatively, sclerosants can be instilled into the pleural cavity, including antibiotics (e.g. doxycycline), antiseptics (e.g. iodopovidone), anticancer drugs or minerals such as talc. Talc pleurodesis is the most common technique, and the talc may either be instilled as a slurry through a small pleural catheter to avoid surgical intervention, or as a dry powder (poudrage) via a surgical approach. Recent work has identified the importance of the particle size of talc in the development of adverse effects from talc pleurodesis.34 Talc particles have been found to enter the lung parenchyma or systemic circulation following pleurodesis, risking the development of pulmonary fibrosis or systemic inflammation respectively. Use of particle sizes greater than 5 μm reduces the complication rate, presumably because the talc particles are unable to pass through the similarly-sized stoma in the pleura (page 445) to gain access to the lymphatics and circulation.
Obliterating the pleura by these techniques may be expected to cause long-term impairment of lung function, but after an initial decline immediately after the procedure, total lung capacity returns to normal approximately 6 months later.35 This is in keeping with the bizarre observation, first discovered in the 1700s, that elephants have no pleural space with connective tissue binding their lungs tightly to the inside of the chest wall, with no apparent long-term ill effects for the species.36
One-Lung Ventilation
Many of the surgical procedures already described will be facilitated by apnoea of the operative lung during surgery. These, and other indications for one-lung ventilation (OLV), are shown in Table 33.1. Indications are divided into absolute, where without OLV the patient’s life is at risk, and relative, when OLV will help manage the patient’s condition but is not mandatory.
Table 33.1 Indications for one-lung ventilation37
| ABSOLUTE INDICATIONS | RELATIVE INDICATIONS |
|---|---|
Isolation of lung to avoid cross contamination
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|