Chapter 29 Pulmonary Infections in Patients with Human Immunodeficiency Virus Disease
Epidemiology, Risk Factors, and Pathophysiology
Human Immunodeficiency Virus Infection: Background
The immune dysregulation that arises from HIV infection means that bacteria, mycobacteria, fungi, viruses, and protozoa can all cause disease in patients with advanced infection. Box 29-1 shows the organisms that typically infect the lung in HIV disease. Of these, the agents of bacterial infections, tuberculosis, and PCP are the most important. In the West, 40% of diagnosed AIDS cases are due to PCP. This chapter provides a brief general overview of the epidemiology and pathogenesis of HIV infection, followed by a more detailed discussion of other important aspects of the disease and its infectious pulmonary complications.
Box 29-1
Etiologic Agents of Human Immunodeficiency Virus (HIV)-Related Pulmonary Infections
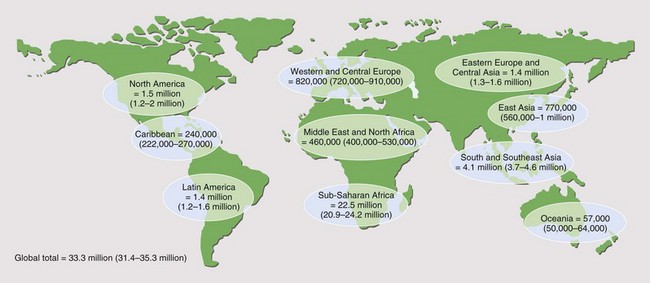
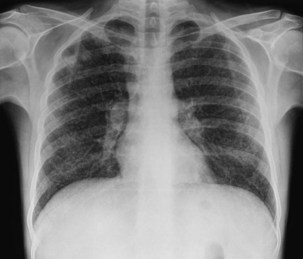
It is reported that by the end of 2009, 33.3 million people worldwide had acquired HIV infection (Figure 29-1). Of these, over 40% are thought to have developed AIDS (for definition of AIDS, see Tables 29-1 and 29-2 and Box 29-2). Globally, 2.6 million people acquired HIV infection in 2009, and 1.8 million died of AIDS. The developing world has been most affected. Sub-Saharan Africa is the current epicenter of the pandemic (accounting for two thirds of all infections); here, nearly 6% of adults are HIV-infected. South and Southeast Asia are responsible for almost a fifth of the estimated HIV global burden. In Central-Eastern Europe and Central Asia, there are currently 1.4 million HIV-infected persons. In the developed world, North America and Western Europe account for approximately 1.5 million and 820,000 infections, respectively. The vast majority of these are spread through sexual contact, although vertical (mother-to-child) and blood-borne infections are common. In the developing world, heterosexual transmission is the norm. In North America and Europe, men who have sex with men constitute the largest group of HIV-infected persons.
Table 29-1 CDC Classification of HIV Infection
| Group | Infection |
|---|---|
| I | Acute primary |
| II | Asymptomatic |
| III | Persistent generalized lymphadenopathy |
| IV | Other disease |
| Subgroup A | Constitutional disease (e.g., weight loss >10% of body weight or >4.5 kg; fevers with temperatures >38.5° C for >1 month; diarrhea lasting >1 month) |
| Subgroup B | Neurologic disease (e.g., HIV encephalopathy, myelopathy, peripheral neuropathy) |
| Subgroup C | Secondary infectious diseases |
| Subgroup C1 | AIDS-defining secondary diseases (e.g., Pneumocystis jirovecii pneumonia, cerebral toxoplasmosis, cytomegalovirus retinitis) |
| Subgroup C2 | Other specified secondary infectious diseases (e.g., oral candidiasis, multidermatomal varicella zoster) |
| Subgroup D | Secondary cancers (e.g., Kaposi sarcoma, non-Hodgkin lymphoma) |
| Subgroup E | Other conditions (e.g., lymphoid interstitial pneumonitis) |
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Table 29-2 CDC Classification System for Human Immunodeficiency Virus (HIV) Infection: Clinical Categories*

Box 29-2
Adult AIDS Indicator Diseases*
Candidiasis of esophagus, trachea, bronchi, or lungs
Coccidioidomycosis, disseminated or extrapulmonary
Cryptococcosis, extrapulmonary
Cryptosporidiosis, with diarrhea for longer than 1 month
Cytomegalovirus disease (not in liver, spleen, or lymph nodes)
Encephalopathy caused by HIV (AIDS dementia complex)
Herpes simplex: ulcers for >1 month, or bronchitis, pneumonitis, esophagitis
Histoplasmosis, disseminated or extrapulmonary
Isosporiasis, with diarrhea for >1 month
Lymphoma: Burkitt, or immunoblastic, or primary in CNS
Mycobacterium avium complex or Mycobacterium kansasii, disseminated or extrapulmonary
Mycobacterium tuberculosis, any site (pulmonary or extrapulmonary)
Mycobacterial infections due to other species or unidentified species, disseminated or extrapulmonary
Pneumocystis jirovecii pneumonia
Pneumonia recurrent within a 12-month period
Progressive multifocal leukoencephalopathy
Salmonella (nontyphoidal) septicemia, recurrent
Natural History of Human Immunodeficiency Virus Infection
Chronic Human Immunodeficiency Virus Infection
The term AIDS was originally created as an epidemiologic tool to capture specific clinical presentations, which early in the HIV epidemic appeared to suggest significant immune deficiency. Over the past 30 years, the definition has been modified to incorporate the expanding spectrum of diseases affecting HIV-infected patients, including cervical carcinoma and recurrent bacterial pneumonia (see Box 29-2). The 1993 CDC classification included an immunologic criterion for AIDS (CD4+ count below 200 cells/µL or CD4+ percentage less than 14% of total lymphocytes) regardless of clinical symptoms (see Table 29-2). These data are used to define a point at which the risk for severe opportunistic infection rises dramatically.
Clinical Features
Bacterial Infection
Bronchiectasis
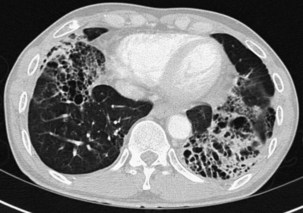
Bronchiectasis is increasingly recognized in HIV-infected patients with advanced HIV disease and low CD4+ lymphocyte counts. It probably arises secondary to recurrent bacterial, mycobacterial or P. jirovecii infections. The diagnosis most often is made by high-resolution (thin-section) computed tomography (CT) scanning (Figure 29-2). Its prevalence has not been accurately determined, although with improved survival from both opportunistic infections and HIV disease, it can be expected to be increasingly common in clinical practice. The pathogens isolated in patients with bronchiectasis are those seen in bronchitis. In addition, Burkholderia cepacia and Moraxella catarrhalis have been described.
Pneumonia
Community-acquired bacterial pneumonia occurs more frequently in HIV-infected patients than in the general population. It is especially common in HIV-infected injecting drug users. The spectrum of bacterial pathogens is similar to that in non–HIV-infected persons (see Box 29-1). S. pneumoniae is the most commonly identified pathogen, followed by H. influenzae. HIV-infected patients with S. pneumoniae–related pneumonia frequently are bacteremic. In one study, the rate of pneumococcal bacteremia in HIV-infected patients was 100 times that for an HIV-negative population. More recent work has confirmed this to be the case for all causes of HIV-related bacterial pneumonia. Typically, blood cultures have a 40-fold increased pick-up rate in HIV-positive patients. The widespread use of ART has led to some decrease in rates of bacterial pneumonia and bacteremia, although they are still considerably higher than in a non–HIV-infected population.
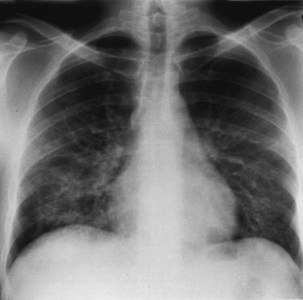
Bacterial pneumonia has a similar presentation in HIV-infected patients and in uninfected persons. Chest radiographs frequently are atypical in appearance, mimicking that in PCP in up to half of the cases (Figure 29-3). By contrast, radiographic lobar or segmental consolidation also may be seen in a wide range of bacterial organisms (Figure 29-4); these include S. pneumoniae, P. aeruginosa, H. influenzae, and M. tuberculosis. PCP also may manifest with lobar or segmental consolidation. In patients with more advanced HIV disease and low CD4+ lymphocyte counts, P. aeruginosa and S. aureus also can cause pneumonia.
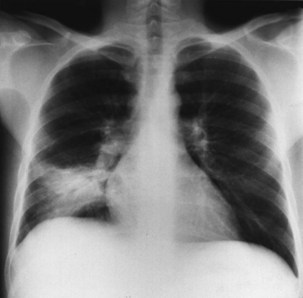
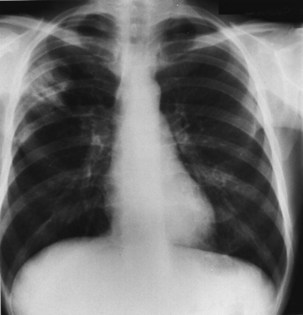
Figure 29-4 Chest radiograph showing lobar consolidation. The etiologic agent was Salmonella choleraesuis.
Mycobacterial Infections
Tuberculosis
Pulmonary disease is the most common presentation, and clinical manifestations are determined by the patient’s level of immunity. For example, persons with reasonably well-preserved CD4+ counts exhibit clinical features similar to those of “normal” adult postprimary disease (Table 29-3). Signs and symptoms typically include weight loss, fever with sweats, cough, sputum, dyspnea, hemoptysis, and chest pain. These patients may have no clinical features to suggest associated HIV infection. The chest radiograph frequently shows upper lobe consolidation, and cavitary change is common (Figure 29-5). If performed, the tuberculin skin test (TST), using purified protein derivative (PPD), usually gives a positive result, and the likelihood that spontaneously expectorated sputum or BAL fluid will be smear-positive for acid-fast bacilli is high.
Table 29-3 Tuberculosis and Human Immunodeficiency Virus (HIV) Infection
| Diagnostic Feature | Stage of HIV Disease | |
|---|---|---|
| Reasonable Immunity | Impaired Immunity | |
| Chest radiographic appearance | Upper zone infiltrates and cavities (cf postprimary infection) | Lymphadenopathy, effusions, miliary or diffuse infiltrates (cf primary infection) Normal |
| Sputum or bronchoalveolar lavage “smear-positive” | Frequently | Less commonly |
| Disease site | Localized | Widely disseminated |
| Tuberculin test–positive | Frequently | Less commonly |
In persons with advanced HIV disease (i.e., low CD4+ lymphocyte counts and clinically apparent immunosuppression), it may be difficult to diagnose tuberculosis. The clinical presentation here often is with nonspecific symptoms. Fever, weight loss, fatigue, and malaise may be mistakenly ascribed to HIV infection itself. In this context, pulmonary tuberculosis is often similar to primary infection, with the chest radiograph showing diffuse or miliary-type shadowing (Figure 29-6), hilar or mediastinal lymphadenopathy, or pleural effusion; cavitation is unusual, with no upper zone chest radiographic predominance. In up to 10% of patients, the chest radiograph may appear normal; in others, the pulmonary infiltrate can be bilateral, diffuse, and interstitial in pattern, thus mimicking PCP. Hilar lymphadenopathy and pleural effusion also may be manifestations of pulmonary Kaposi sarcoma or lymphoma, with which M. tuberculosis may coexist. The TST result usually is negative, and spontaneously expectorated sputum and BAL fluid samples often are smear-negative (although they are culture-positive).
In addition to pulmonary tuberculosis, extrapulmonary disease occurs in a high proportion of HIV-infected persons with low CD4+ lymphocyte counts (less than 150 cells/µL). Mycobacteremia and generalized lymph node infection (Figure 29-7) are common, but involvement of bone marrow, liver, pericardium, meninges, and brain also has been described.
Infections Due to Mycobacteria Other Than Tuberculosis
Pneumocystis jirovecii Pneumonia
The clinical presentation of PCP is nonspecific, with onset of progressive exertional dyspnea over days or weeks, together with a dry cough, with or without expectoration of minimal quantities of mucoid sputum. Patients often report an inability to take a deep breath, which is not due to pleurisy (Table 29-4). Fever is common, yet patients rarely complain of temperature-related signs and symptoms including sweats. In HIV-infected patients, the presentation usually is more insidious than in those receiving immunosuppressive therapy. The median time to diagnosis from onset of symptoms is more than 3 weeks in those with HIV infection, compared with less than 1 week in non–HIV-infected patients. In a small proportion of HIV-positive patients, the disease course of PCP is fulminant, with an interval of only 5 to 7 days between onset of symptoms and progression to development of respiratory failure. In others, it may be much more indolent, with respiratory symptoms that worsen almost imperceptibly over several months. Rarely, PCP may manifest as a fever of undetermined origin without respiratory symptoms.
Table 29-4 Clinical Presentation in Pneumocystis jirovecii Pneumonia
| Examination | Typical Presentation | Atypical Presentation |
|---|---|---|
| Symptoms | Progressive exertional dyspnea over days or weeks | Sudden onset of dyspnea over hours or days |
| Dry cough ± mucoid sputum | Cough productive of purulent sputum Hemoptysis | |
| Difficulty taking in a deep breath not related to pleuritic pain | Chest pain (pleuritic or “crushing”) | |
| Fever ± sweats Tachypnea | ||
| Signs | Normal breath sounds or fine end-inspiratory basal crackles | Wheeze, signs of focal consolidation or pleural effusion |
| Chest radiographic appearance | Early: perihilar “haze,” or bilateral interstitial shadowing | Pleural effusion, lobar or segmental consolidation |
| Late: alveolar-interstitial changes or “whiteout” (marked alveolar consolidation with sparing of apices and costophrenic angles) | ||
| Arterial blood gases | PaO2: early: normal; late: low | |
| PaCO2: early: normal or low; late: normal or high |
Clinical examination usually is remarkable only for the absence of physical signs; occasionally, fine, basal, end-inspiratory crackles are audible. Features that would suggest an alternative diagnosis include a cough productive of purulent sputum or hemoptysis, chest pain (particularly pleural pain), and signs of focal consolidation or pleural effusion (see Table 29-4). Of note, infection with more than one pathogen occurs in almost one fifth of these patients, so symptoms may be related to infection with any of several agents.
The chest radiographic appearance in PCP typically is unremarkable initially. Later, diffuse reticular shadowing, especially in the perihilar regions, is seen and may progress to widespread alveolar consolidation that resembles that in untreated pulmonary edema or with presentation late in disease. At this stage, the lung may be grossly consolidated and almost airless (Figure 29-8). Up to 20% of chest radiographs are atypical in appearance, showing lobar consolidation, honeycomb lung, multiple thin-walled cystic air spaces (pneumatoceles), intrapulmonary nodules, cavitary lesions, pneumothorax, and hilar and mediastinal lymphadenopathy. Predominantly apical changes, resembling those of tuberculosis, may occur in patients with PCP that developed subsequent to anti–P. jirovecii prophylaxis with nebulized pentamidine (Figure 29-9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree