Pulmonary Infection: Basic Concepts
Despite advances in diagnosis and treatment, respiratory tract infection continues to be a major cause of morbidity and mortality. Pneumonia is the leading cause of death due to infectious disease (1). More than 6 million cases of bacterial pneumonia occur each year in the United States and the incidence of pneumonia is increasing. The spectrum of organisms known to cause respiratory infections is broad and constantly increasing as new pathogens are identified, and an increasing number of patients have impaired immunity due to disease or medications. In the United States, it has been estimated that there are 1.1 million cases of community-acquired pneumonia requiring hospitalization each year (1). Nosocomial pneumonia is the most important hospital-acquired infection, being associated with the highest mortality rate of nosocomial infections (2). In addition to direct patient care costs, pneumonia is responsible for >50 million days of restricted activity from work and is the sixth leading cause of death in the United States with a mortality rate of 13.4 per 100,000 (3).
In the last two decades there has been an increase in not only the prevalence of various infections but also the recognition of several important new viral pathogens. These include hantaviruses, human metapneumovirus, avian influenza A viruses, and coronavirus associated with severe acute respiratory syndrome (SARS) (4,5,6,7,8,9,10,11).
Pulmonary Host Defenses
Microorganisms may reach the lower respiratory tract through diverse routes. Although breathed in air contains a myriad of particulate contaminants, some of which are infectious, by far the most common route for bacterial pneumonia is microaspiration from infected oropharyngeal secretions. Aerosolization is an important route of infection for those pathogens believed to be directly inhaled rather than aspirated into the lower respiratory tract, such as Mycobacterium tuberculosis, endemic fungi, Mycoplasma, Legionella, and many respiratory viruses. Gross aspiration occurs in patients with central nervous system disorders affecting swallowing (e.g., seizures, strokes). Hematogenous spread commonly takes place in the setting of endocarditis and intravascular catheter-related infections.
Pulmonary host defense mechanisms include innate or nonspecific (e.g., mechanical barriers and phagocytic defenses) and acquired or specific (e.g., cell-mediated defenses and humoral immunity) mechanisms. Impairment in any of these mechanisms results in reduced ability to clear an infectious inoculum.
Mechanical barriers include the anatomic features of the airways and the mucociliary transport system. Clearance by
mechanical means is influenced by the physical properties of inhaled infectious organisms and particles. Whereas particles >10μm in diameter are filtered in the upper airways (nasopharynx), particles 5 to 10μm in diameter may reach the tracheobronchial tree and are cleared by the mucociliary escalator. Only particles between 1 and 2μm in diameter typically reach the alveoli.
mechanical means is influenced by the physical properties of inhaled infectious organisms and particles. Whereas particles >10μm in diameter are filtered in the upper airways (nasopharynx), particles 5 to 10μm in diameter may reach the tracheobronchial tree and are cleared by the mucociliary escalator. Only particles between 1 and 2μm in diameter typically reach the alveoli.
After reaching the distal portions of the lung the ability of infectious organisms to cause progressive infection depends on the balance between the virulence and load of the organism and phagocytic lung defenses (12). Phagocytic functions in the lung are carried out by mononuclear (monocytes, macrophages) and polymorphonuclear (neutrophils, eosinophils) cells (13).
Alveolar macrophages (“big eaters”) represent the first line of defense at the level of the alveoli. Macrophages derive from precursors in the bone marrow, and are mobilized to the active focus during active lung infection (13). At the alveolar level, phagocytes are recruited from the interstitium, airways, and blood. Alveolar macrophages have several functions that are important to the host response to bacteria, including bacterial recognition, bacterial phagocytosis, bacterial killing, and the production of inflammatory mediators that are essential for the pulmonary recruitment of leukocytes (13).
Macrophages and monocytes recognize bacteria with a set of cell surface receptors known as pattern recognition receptors. Recognition of bacteria by these receptors results in macrophage/monocyte activation and the development of inflammatory response to bacterial pathogens (14,15).
Impaired alveolar macrophage function occurs in various conditions such as hypoxia, alcoholism, tobacco smoke, and corticosteroid therapy. It is estimated that 20 million individuals in the United States meet the criteria for alcoholism, and between 20% and 40% of patients admitted to large urban hospitals are there because of disease caused by or made worse by alcohol consumption (16,17). A Canadian group studying pneumonia in >6,000 alcoholics reported a three- and sevenfold increased risk of death in ethanol-abusing men and women, respectively, compared to controls (18).
Failure of mechanical mechanisms such as the mucociliary escalator and phagocytic defenses favors the generation of a humoral immune response specifically directed toward the elimination of extracellular pathogens. The development of specific immune responses in the lungs requires presentation of antigen to CD4+ T lymphocytes.
Pneumonia is more common when host defense is impaired. Defects in phagocytosis or ciliary function, hypogammaglobulinemia, neutropenia, and reduction in CD4+ T lymphocytes can result in increased frequency and severity of pneumonia. In immunocompromised patients, defects in the different components of the immune system predispose the patient to develop specific types of infections (19). Therefore awareness of the immune status of the patient and any underlying abnormality that may affect the immune response is important in the differential diagnosis. For example, functional or anatomic asplenia is an important risk factor for pneumonia, with 80% of cases being due to Streptococcus pneumoniae.
Changing Trends in Pulmonary Infections
Diagnosis of pneumonia requires clinical acumen, appropriate microbiologic tests, and imaging. The chest radiograph represents an important initial examination in all patients suspected of having pulmonary infection. In most cases the radiographic findings are suggestive of or consistent with the diagnosis of pneumonia and are sufficiently specific in the proper clinical context to preclude the need for additional imaging (20,21,22,23).
The clinician evaluating the patient with a known or suspected diagnosis of pulmonary infection faces a diagnostic challenge because the infection may be caused by a variety of organisms that may present with similar clinical symptoms and signs and result in similar radiographic manifestations. Furthermore, the radiographic manifestations of a given organism may be variable depending on the immunologic status of the patient and the presence of pre- or coexisting lung disease.
The number of immunocompromised patients has increased considerably in the last three decades because of three main phenomena: The acquired immunodeficiency syndrome (AIDS) epidemic, advances in cancer chemotherapy, and expanding solid organ and hematopoietic stem cell transplantation. At the onset of the AIDS epidemic in the early and mid-1980s, there was 50% to 80% mortality for each episode of Pneumocystis pneumonia (PCP). Since routine prophylaxis was instituted in 1989, there has been a declining incidence of PCP in the AIDS population (24,25,26) and a decrease in mortality in mild to moderate cases (27). However, other infections including bacterial pneumonia, fungal infection, cytomegalovirus (CMV), Mycobacterium Avium-intracellulare complex (MAC), and tuberculosis remain a significant cause of morbidity and mortality in these patients (24,25,26,27). The role of imaging is to identify the presence, location, and extent of pulmonary abnormalities, the course and evolution of pneumonia, the presence of associated complications, and detection of additional or alternative diagnosis.
Integrating Clinical and Imaging Findings
The most useful imaging modalities for the evaluation of patients with known or suspected pulmonary infection are chest radiography and computed tomography (CT). Imaging examinations should always be interpreted with awareness of the clinical findings including duration of symptoms, presence of fever, cough, dyspnea,
and the presence or absence of leukocytosis (28). Knowledge of whether the patient has community-acquired or nosocomial pneumonia, as well as knowledge of the immune status of the patient, are most helpful in the differential diagnosis and determination of the most likely causative organisms (28,29). Clinical information can greatly enhance the accuracy of the radiographic diagnosis. For example, the AIDS patient with an acute airspace process who has chills, fever, and purulent sputum probably has pyogenic rather than a Pneumocystis pneumonia. In the absence of clinical information, radiologists cannot reliably distinguish between pneumonia and other pulmonary processes (30). Unfortunately, the clinical data and radiographic findings often fail to lead to a definitive diagnosis of pneumonia because there is an extensive number of noninfectious processes associated with febrile pneumonitis, including drug-induced pulmonary disease, acute eosinophilic pneumonia, organizing pneumonia (bronchiolitis obliterans organizing pneumonia [BOOP]), and pulmonary vasculitis that may mimic pulmonary infection (31).
and the presence or absence of leukocytosis (28). Knowledge of whether the patient has community-acquired or nosocomial pneumonia, as well as knowledge of the immune status of the patient, are most helpful in the differential diagnosis and determination of the most likely causative organisms (28,29). Clinical information can greatly enhance the accuracy of the radiographic diagnosis. For example, the AIDS patient with an acute airspace process who has chills, fever, and purulent sputum probably has pyogenic rather than a Pneumocystis pneumonia. In the absence of clinical information, radiologists cannot reliably distinguish between pneumonia and other pulmonary processes (30). Unfortunately, the clinical data and radiographic findings often fail to lead to a definitive diagnosis of pneumonia because there is an extensive number of noninfectious processes associated with febrile pneumonitis, including drug-induced pulmonary disease, acute eosinophilic pneumonia, organizing pneumonia (bronchiolitis obliterans organizing pneumonia [BOOP]), and pulmonary vasculitis that may mimic pulmonary infection (31).
Distinction of localized pneumonia from other pulmonary processes cannot be made with certainty on radiologic grounds (31,32). Localized pulmonary disease of a lobar or segmental distribution can be produced not only by pneumonia but also by obstructive pneumonitis, hemorrhage, or aspiration of sterile gastric contents. Diagnosis is equally difficult when pneumonia appears as a diffuse pulmonary abnormality. Extensive bilateral abnormalities may be due to bronchopneumonia or due to hydrostatic pulmonary edema, acute respiratory distress syndrome (ARDS), or diffuse pulmonary hemorrhage (33,34,35).
Chest Radiography
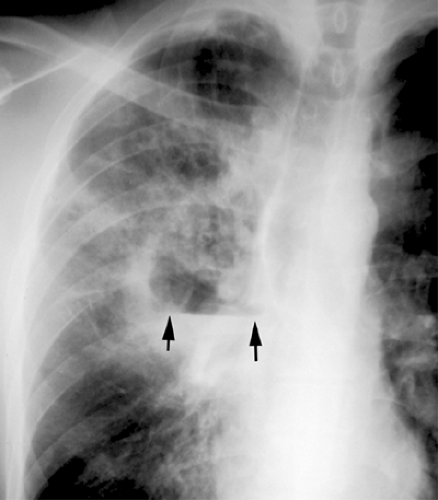
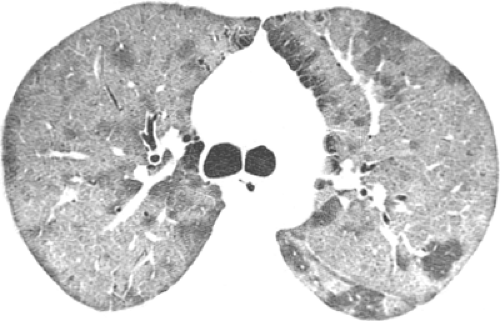
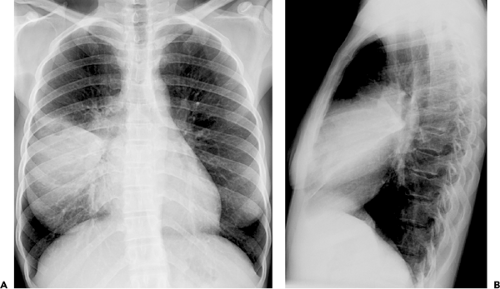
The American Thoracic Society guidelines recommend that posteroanterior (PA) (and lateral when possible) chest radiographs be obtained whenever pneumonia is suspected in adults (36). The role of chest radiography is as a screening tool for the detection of abnormalities consistent with pneumonia and for monitoring response to therapy. Other roles for chest radiography include assessment of disease extent, detection of complications (i.e., cavitation, abscess formation, pneumothorax, and pleural effusion), detection of additional or alternative diagnoses and, in some cases, guiding invasive diagnostic procedures (see Fig. 1.1).
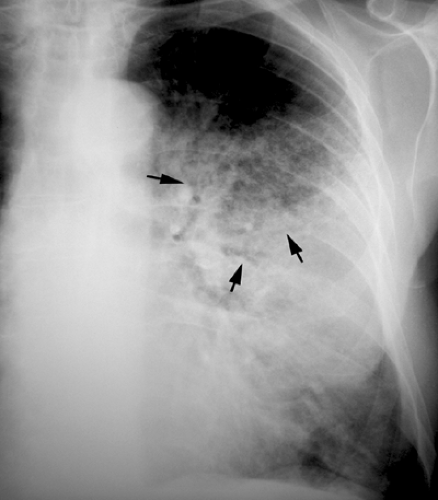
The most common radiographic manifestations of respiratory infection are foci of consolidation, ground-glass opacities, or reticulonodular opacities (see Fig. 1.2). Other less common radiographic findings include hilar and mediastinal lymphadenopathy, pleural effusion, cavitation, and chest wall invasion. These findings are not specific and may be seen in other conditions. Furthermore any given organism may result in a variety of different patterns of presentation. For example, Pneumocystis may result in bilateral ground-glass opacities or consolidation, or, less commonly, focal consolidation, nodules, miliary pattern, or reticulation (36). In up to 10% of patients with proved PCP the chest radiograph is normal (37).
Computed Tomography
Computed tomography (CT) is a useful adjunct to conventional radiography in selected cases (30,32,38,39). There is a large literature indicating that CT scan is a sensitive method capable of imaging the lung with excellent spatial resolution and providing anatomic detail similar to that seen by gross pathologic examination. Differences in tissue attenuation and parenchymal changes caused by an acute inflammatory process can be seen readily on CT scan (38,39). CT scan can also be helpful in the detection, differential diagnosis, and management of patients with pulmonary complications.
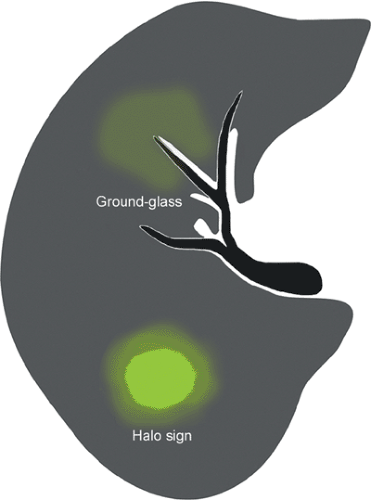
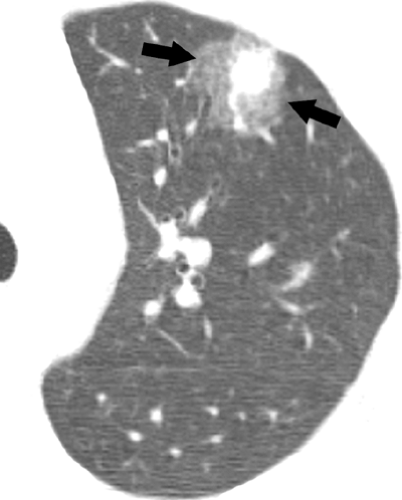
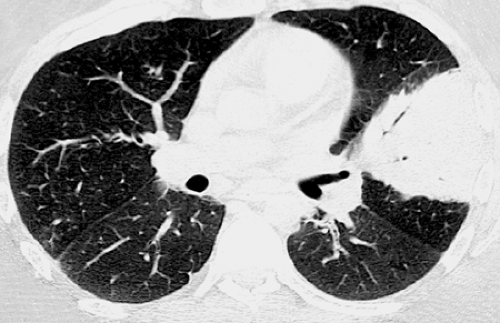
Optimal assessment of the parenchyma is obtained with the use of high-resolution CT scan, which allows assessment of the pattern and distribution of abnormalities down to the level of the secondary pulmonary lobule (38). The findings of airspace disease, including airspace nodules, ground-glass opacities, consolidation, air bronchograms, and centrilobular or perilobular distribution, are seen better in CT scan than in conventional radiography (37,38). Airspace nodules measure 6 to 10 mm in diameter and usually reflect the presence of peribronchiolar consolidation, and therefore are centrilobular in distribution. They are best appreciated in early disease and best seen at the edge of the pathologic process in which consolidation is incomplete. In some circumstances, nodules may be associated with a “halo” of ground-glass attenuation (see Fig. 1.3). In severely neutropenic patients this “halo” sign is highly suggestive of angioinvasive aspergillosis (40) (see Fig. 1.4). However, a similar appearance has been described in other conditions including infection by nontuberculous mycobacteria, Mucorales, Candida, herpes simplex virus, CMV, Wegener granulomatosis, Kaposi sarcoma, and hemorrhagic metastases (41).
Ground-glass opacity is defined as hazy increased lung opacity that does not obscure the underlying vascular structures (Fig. 1.3). Ground-glass opacities are a common but nonspecific high-resolution CT scan finding that may result from a variety of interstitial and airspace diseases. Infections that typically present with bilateral ground-glass opacities are Pneumocystis and CMV pneumonia (see Fig. 1.5). In AIDS patients the presence of extensive bilateral ground-glass opacities is highly suggestive of PCP. In immunocompromised non-AIDS patients the differential diagnosis includes CMV pneumonia, drug-induced lung disease, pulmonary hemorrhage and organizing pneumonia (42).
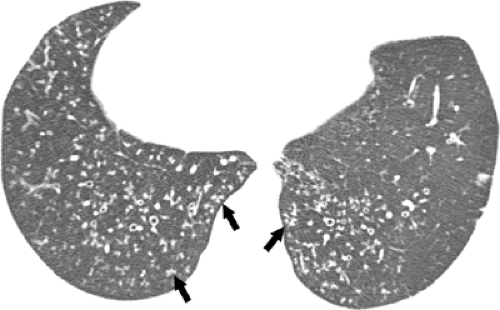
A “tree-in-bud” pattern is a characteristic high-resolution CT scan manifestation of infectious bronchiolitis (see Fig. 1.6). It consists of centrilobular branching tubular and nodular structures and reflects the presence of bronchiolar inflammation and filling of the lumen by inflammatory material or mucus (43). This pattern may be seen in a variety of bacterial, mycobacterial, fungal, and viral infections (see Fig. 1.7) (43,44).
Airspace consolidation, defined as a localized increase in lung attenuation that obscures the underlying vascular structures, may be seen in association with bacterial, fungal, and viral infections (see Fig. 1.8). Focal areas of consolidation secondary to infection in immunocompromised AIDS and non-AIDS patients are most commonly due to bacterial pneumonia (31). Fungal infection needs to be considered particularly in neutropenic patients with hematologic malignancies (42). Parenchymal disease in mycobacterial infection may also appear as patchy nodular areas of consolidation, with or without cavitation (45).
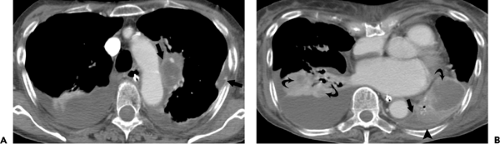
Although CT scan is not recommended for the initial evaluation of patients with pneumonia, it is a valuable adjunct to conventional radiography, being helpful in better characterizing complex pneumonias and in detecting complications (36) (see Fig. 1.9). CT scan is also indicated to rule out underlying lung disease such as lung cancer
or bronchiectasis in patients with recurrent or nonresolving pneumonia. Several studies have shown that high-resolution CT scan is particularly helpful in the detection, differential diagnosis, and management of immunocompromised patients with pulmonary complications (36,37,38,39). These studies have also shown that CT scan may confirm the presence of pneumonia in patients with clinical symptoms and normal or questionable radiographic findings.
or bronchiectasis in patients with recurrent or nonresolving pneumonia. Several studies have shown that high-resolution CT scan is particularly helpful in the detection, differential diagnosis, and management of immunocompromised patients with pulmonary complications (36,37,38,39). These studies have also shown that CT scan may confirm the presence of pneumonia in patients with clinical symptoms and normal or questionable radiographic findings.
Community-Acquired Pneumonia
Community-acquired pneumonia is a major health care problem because of associated morbidity and mortality (1,3). The overall rate of pneumonia ranges from 8 to 15 per 1,000 persons per year, with the highest rates at the extremes of age and during winter months (46). Between 485,000 and 1 million patients each year are hospitalized in the United States for treatment of community-acquired pneumonia. The costs of inpatient care exceed outpatient care by a factor of 15 to 20, and comprise most of the estimated $8.4 billion spent annually for care of patients with pneumonia (1,3,47,48).
Hospital admission rates of pneumonia episodes vary from 22% to 51% of patients with community-acquired pneumonia (1). The mortality is higher in less-developed countries, as well as in young and elderly patients. Pulmonary opacities are usually evident on the radiograph within 12 hours of the onset of symptoms. Although radiographic findings do not allow a specific etiologic diagnosis, the radiograph may be helpful in narrowing down the differential diagnosis. In community-acquired pneumonia, diagnosis and disease management most frequently involve chest radiography and generally do not require the use of other imaging modalities (49).
TABLE 1.1 Differential Diagnosis of Community-Acquired Pneumonia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The spectrum of causative organisms of community-acquired pneumonia includes gram-positive bacteria such as S. pneumoniae (Pneumococcus), Haemophilus influenzae, and Staphylococcus aureus, as well as atypical organisms such as Mycoplasma pneumoniae, Chlamydia pneumoniae, or Legionella pneumophila and viral agents such as influenza A virus and respiratory syncytial viruses (see Table 1.1). S. pneumoniae is by far the most common cause of complete lobar consolidation (50,51,52) (see Fig. 1.10). Other causative agents that produce complete lobar consolidation include Klebsiella pneumoniae and other gram-negative bacilli, L. pneumophila, H. influenzae, and occasionally M. pneumoniae (50,51,52,53).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree