Fig. 14.1
Diagnostic algorithm of PH
Classification of Pulmonary Hypertension
The World Health Organization (WHO) has condensed all forms of PH into five main groups [8] (Table 14.1). Group I in the WHO classification includes all forms of pulmonary arterial hypertension (PAH), (Idiopathic (IPAH), Familial (FPAH) and Associated (APAH)). These forms of PH were formerly recognized as pre-capillary PH because the increase in PAP is mainly attributable to a sustained increase in the arteriolar tone. For diagnosing PAH, in addition to the above-cited criteria, pulmonary wedge pressure (PWP- that is the pressure measured by wedging a pulmonary catheter with an inflated balloon into a small pulmonary arterial branch) should be ≤15 mmHg and the pulmonary vascular resistance (PVR) >3 Woods Units [2]. Group II includes the vast majority of cases of PH, namely those associated with the presence left heart disorders. In these patients PWP is >15 mmHg. As the arteriolar tone is usually normal or just slightly increased, PH forms in this group were formerly defined as post-capillary. Group III and IV comprises any form of PH consequent to lung diseases and/or hypoxia and chronic thromboembolism, respectively. All forms of PH with unclear or multifactorial etiologies are eventually labeled as Group V PH.
Table 14.1
WHO classification of pulmonary hypertension (PH)
Group | Definition | Conditions |
|---|---|---|
I | Pulmonary arterial hypertension (PAH) | Congenital heart disease, connective tissue diseases, drugs and toxins, HIV infection, portal hypertension, pulmonary veno-occlusive disease |
Idiopathic (IPAH) | ||
Familial (FPAH) | ||
Associated (APAH) | ||
II | PH associated to left heart disorders | Left heart systolic dysfunction, left heart diastolic dysfunction, left-sided valve disease (mitral and/or aortic) |
III | PH associated to lung diseases and/or hypoxia | Chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), sleep apnea |
IV | PH associated to chronic thromboembolism | Obstruction of pulmonary arterial vessels (proximal or distal) by thromboemboli, tumors, or foreign bodies |
V | PH of unclear or multifactorial etiology | Renal, hematologic, systemic and metabolic disorders |
Epidemiology of PH
Prevalence of PH in the General Population
In last years, evidence has been accumulated showing that mild to moderate forms of PH are much more common than usually supposed [7]. PH often remain undetected because of the long, preclinical asymptomatic phase and mostly suspected only when the clinical signs and symptoms of right ventricular dysfunction (dyspnea, fatigue, non-productive cough, angina pectoris, syncope, peripheral edema and, rarely, hemoptysis) appear [2]. In the Olmsted county study [9], a general population study conducted in a random sample of the same county, the prevalence of PH defined by a Doppler-derived PASP >35 mmHg in individuals older than 45 years was about 5 %. Most cases of PH detected in this population study were secondary to concomitant heart disorders, particularly those associated with LV function impairment. The presence of PASP was predicted by diastolic dysfunction (as measured by the E/e′ ratio (early trans-mitral flow velocity [E] to early mitral annular tissue velocity [e′]) and by the presence of systemic hypertension and high pulse pressure.
Pre-capillary PH (PAH, WHO Group I) is much more rare with an estimated prevalence of about 15 cases per million and an annual incidence of about 2–3 per million [7]. Adult females are almost three times as likely to present with PAH than adult males. In children, the presentation of PAH is more evenly split along gender lines. As mentioned, WHO group 1 includes a miscellany of forms spanning from PH associated with connective tissue diseases, drugs and various toxic agents sporadic and idiopathic forms. Conversely, the highest prevalence of PH (30 %) is associated with congenital heart disease [10] and sleep breathing disturbances (15–20 %) [11] while a lower prevalence has been reported in systemic sclerosis (7–12 %) [12, 13] and in portal hypertension (2–6 %) [14, 15].
Prevalence of PH in Chronic Kidney Disease
In chronic kidney disease (CKD) patients, it is now widely accepted that PH is a problematic condition not only confined to connective tissue and systemic diseases and that the impairment in kidney function may elicit per se the development and/or worsening of this condition. Epidemiological data on PH in CKD patients are scarce and sparse and mainly based on retrospective data. This hampers the possibility to provide precise estimations on the overall prevalence of PH in the CKD population. Furthermore, in only one study [16] PASP has been measured by right atrial catheterization, the gold-standard method indicated by current guidelines, while in the remainder PASP was estimated by Echo-Doppler with poor or no uniformity in the methodology implemented. Different PASP cut-offs (ranging from 25 to ≥45 mmHg) were considered as indicative of the presence of PH [17–36] and in one study a Vmax ≥2.5 m/s was assumed as the major diagnostic criterion [37]. Such a variability in the diagnostic criteria adopted by these studies explains the reported wide range of PH prevalence in CKD patients and limits the possibility to perform crude comparisons between studies.
No data on the prevalence of PH in early CKD stages [1–3] are currently available. Among pre-dialysis patients with CKD stage 5 (CKD-5 ND), the prevalence of PH is about two to eight times higher than in the general population, ranging from 9 to 39 % [17, 19–22]. PH prevalence is higher in the dialysis population (CKD-5D) than in CKD-5 ND patients. In the only study measuring PAP by invasive methods [16], PH was present in 81 % of HD and 71 % of CKD stage 4–5 patients. In this selected population, the prevalence of (pre-capillary) pulmonary artery hypertension (WHO Group I) was 6 % in CKD stage 4–5 patients and 13 % in HD patients and the prevalence of WHO Group-II PH was 71 and 65 % respectively. With regard to dialysis modality, the prevalence of PH is lower in patients on peritoneal dialysis (from 0 to 42 %) than in hemodialysis patients (from 18.8 to 68.8 %) [23, 28–31, 33–36]. The presence of artero-venous fistula (AVF) in HD patients has been hypothesized as one of the possible explanations underlying this difference (see below). In studies directly comparing HD and PD patients of the same center, PH was notably less prevalent in PD patients [21, 23, 28, 31]. Restricted evidence on PH in CKD has been accrued in western countries [19, 30–34, 37] while the majority of investigations were performed in the Middle East [17, 18, 20–29, 35]. In five studies performed in the US [19, 32–34, 37] the prevalence of PH ranged from 25 to 47 %. This prevalence resulted more homogenous (32–42 %) in the four studies that referred to the same diagnostic PASP cut-off (≥35 mmHg) [19, 32–34].
Risk Factors for PH in CKD Patients
As for PH in the general population, the vast majority of factors responsible for PH in CKD patients still remains poorly defined. Because most cases of PH in CKD patients are post-capillary in nature (WHO group II) [16], these forms are likely to depend mostly on the presence of associated LV disorders, which are quite prevalent in CKD and, particularly in HD patients. Even though the impairment of left heart plays a key role in the genesis of PH, yet most CKD patients often present with further conditions able to induce and/or exacerbate PH with mechanism(s) acting at the pre-capillary level (Fig. 14.2).
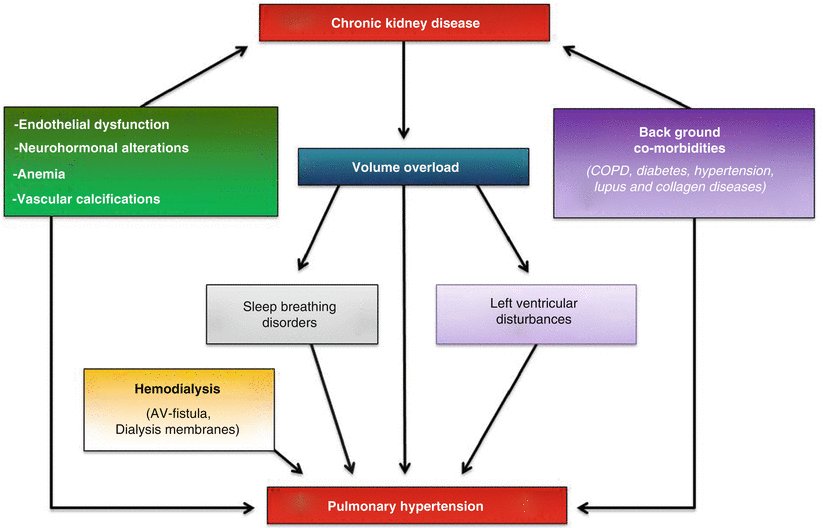

Fig. 14.2
Complex interplay mechanism among CKD and other risk factors in determining PH
Artero-venous Fistula
The presence of artero-venous fistulas (AVF) may in part explain the higher prevalence of PH among HD patients undergoing chronic replacement therapy than in PD or in CKD patients not on dialysis [18, 28, 31]. Evidences in HD patients indicate a rise in pulmonary pressures in strict parallelism with AVF creation [38]. Furthermore, PH tends to worsen overtime in the HD population [22, 31] and AVF-flow and AVF-duration are independently correlated with the severity of PH [20]. Temporary AVF compression by a sphygmomanometer [18, 21, 24] or surgical AVF closure [39] are both able to induce a rapid decrease in the mean cardiac output followed by a stable decline in pulmonary pressures. Many hypotheses have been proposed to explain the role of AVF in the pathogenesis and worsening of PH. AVF, be them traumatic or intentionally created, is known to exert profound hemodynamic effects such as decreased systemic vascular resistances, enhanced venous return and increased cardiac output to maintain proper blood flow to all organs and tissues. These adaptations might increase pulmonary blood flow and prepare the ground for pulmonary hypertension. Because pressure is the product of flow and resistance, at any level of pulmonary vascular resistance, increased pulmonary flow necessarily leads to increased pressure. Although important, the presence of AVF alone fails to explain the highest prevalence of PH observed in the dialysis population. The demonstration that kidney transplantation may revert to normal pulmonary artery pressure in patients who still have a functioning AVF [18] suggests that other factors than AVF might play an equally significant role.
Endothelial Dysfunction
Endothelial dysfunction – that is a systemic pathological state characterized by an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) the endothelium- is a major determinant of PH [40] and is highly pervasive in CKD patients, especially in dialysis patients [41]. The hypothesis that endothelial dysfunction might play a central role in the genesis of PH in HD patients is supported by cross-sectional findings showing that plasma levels of nitric oxide (NO, a powerful vasodilator) are reduced in HD patients with PH as compared to those without PH and by the observation that in patients without PH, HD treatment increases NO levels to a greater extent than in those with abnormal pulmonary resistances [24]. Asymmetric dimethyl-arginine (ADMA), an endogenous inhibitor of NO synthase which is copiously synthesized at lung level [42], has been strongly involved in experimental [43] and in primary forms [44] of PH. Since ADMA attains very high concentrations in subjects with renal function impairment [45], this uremic toxin might be considered as an additional factor potentially involved in PH in this population.
Sleep Breathing Disorders
Episodes of nocturnal hypoxia are frequent in both pre-dialysis [46] and dialysis CKD patients [47]. Chronic nocturnal hypoxia is the key pathophysiological effect of a wide spectrum of sleep breathing disorders, including sleep apnea. Nocturnal hypoxemia by sleep apnea, in turn, is a strong trigger of PH in experimental models [48] and a close link between oxygen saturation and pulmonary artery pressure has been established in experimental studies in healthy humans and in patients with chronic lung diseases [49]. In CKD patients, volume overload is the major trigger of sleep apnea. Experimental studies suggest that hypoxemia increases pulmonary pressure by enhancing sympathetic activation [50]. Interestingly, ADMA is increased in patients with sleep breathing disorders [51]. Furthermore, circulating levels of ADMA in CKD patients go along with sympathetic nerve activity measured in the peroneal nerve [52] and with norepinephrine levels in dialysis patients [53]. Given the strong vasoconstriction potential of ADMA in the lung vasculature and the observation that sympathetic system activity and ADMA share a common pathogenic pathway leading to left ventricular hypertrophy and to cardiovascular events in CKD patients [54], it might be possible that this pathway is also somewhat implicated in the genesis of PH in the CKD population.
Exposure to Dialysis Membranes
During HD sessions, blood membrane contact and reversible neutrophil sequestration in the lung causes neutrophils activation [55]. This may contribute to cause and/or worsen microvascular lung disease in HD patients. Neutrophil activation is much marked with cellulosic membranes and it is much attenuated, although not abolished, with modified cellulosic and synthetic membranes. In a crossover trial in a series of 74 patients the use of high flux polysulphone filters was associated with a more pronounced fall in post dialysis pulmonary pressure than the use of cellulose acetate filters [35].
Systemic Diseases Associated with CKD and Other Risk Factors
In CKD patients the control of microvascular tone in the lung might also be affected by several pre-existing connective tissue diseases and superimposed liver, infectious and hematologic diseases. Even though all these conditions may contribute to PH in CKD patients however, collectively, these factors fail to explain the high prevalence of PH associated with CKD because most patients display PH even in the absence of these diseases [56]. Severe anemia is an established cardiovascular risk factor in CKD and its impact on the cardiovascular system includes direct effects to the pulmonary circulation. Low hemoglobin levels could contribute to PH by aggravating hypoxia triggered by concomitant conditions [57]. As a part of a systemic deregulation in mineral metabolism, arterial rigidity is increased in CKD and calcium deposits can be demonstrated in the pulmonary artery in kidney disease, thus implicating arterial stiffness in PH in this population [58]. Indeed, in the Olmsted study PASP was directly related to pulse and systolic pressure as well as to age, suggesting that stiffening of the pulmonary artery may play a role in PH at community level [9]. Experimental studies in the dog show that PTH may per se increase pulmonary resistances [59]. Nevertheless, two different studies in CKD patients [24, 25] failed to demonstrate an association between PTH levels and the severity of pulmonary calcifications and PTH levels were not different between patients with or without PH [24].
PH Is a Risk Factor for Worse Outcomes in CKD
PH is a risk factor for cardiovascular morbidity and mortality in the general population and a large US survey, recording data on all forms of PH over a 22 year-period (1980–2002), documented a stable death rate in patients with PH, ranging from 5.2 to 5.4 per 100,000 [60]. Findings in a cohort of 500 patients with PH WHO-Group 1 [61] indicate that the presence of an impaired renal function conveys a higher risk for PH. Furthermore, in the same cohort pathological serum creatinine levels were associated with higher right atrial pressure, lower cardiac index and an increased risk of death. Data about clinical outcomes in CKD-5 patients with PH are scarce. In one study on pre-dialysis CKD patients, the presence of PH (defined as having a Doppler-estimated PASP ≥45 mmHg) was associated with a higher risk (HR 3.6) of death [17]. In dialysis patients undergoing kidney transplantation the improvement in left ventricular geometry and function is usually associated with a parallel improvement in PH [18, 30]. Information on the impact of PH on cardiovascular outcomes in chronic hemodialysis patients is available in five studies [17, 21, 24, 36, 37]. In two reports [17, 21] the same cohort has been studied with a different patients accrual and different diagnostic criteria of PH. In the first analysis performed on 58 patients [21], the presence of a Doppler-estimated PASP ≥35 mmHg was associated with a higher mortality rate (30.8 %) as compared to PASP values ≤35 mmHg (3.5 %). In the second analysis conducted on 127 HD patients PH (ePASP ≥45 mmHg) was an independent risk factor for death (HR 2.4) [17]. In 90 chronic HD patients with AVF [37], mortality was four-fold higher in patients with PH, defined by the presence of TRV ≥2.5 m/s (26 %/year), in comparison to those without (6 %). Furthermore PH was related with impaired left systolic ventricular function and elevated pulmonary capillary wedge pressure. Similarly, in another recent study [34] PH had a 38 % prevalence among 228 chronic hemodialysis patients and carried a high death risk (HR 2.17; 95 % CI 1.31–3.61, P < 0.01) after adjustment for other risk factors. In a Chinese cohort of 278 HD patients [36] the prevalence of PH was reported to be even higher (64.7 %) and PH was an independent predictor of all-cause mortality [HR 1.85; 95 % CI 1.03–3.34] CV mortality [HR 2.36; 95 % CI 1.05–5.31] and CV events [HR 2.27; 95 % CI 1.44–3.58] after multiple adjustment. Interestingly, the presence of PH in 215 HD patients waitlisted for kidney transplant predicted the risk of death after kidney transplantation [19]; this suggests that this procedure may not reverse the excess risk associated with established PH. Whether PH in CKD represents a consequence of concomitant LV disorders with scarce direct impact upon clinical outcomes or whether it represents a truly independent risk factor for death and adverse cardiovascular outcomes still remains an unanswered question. Large prospective studies adopting well standardized criteria including right heart catheterization are eagerly awaited to establish the risk conveyed by the presence of PH in CKD stage-5 ND and in dialysis patients. In particular, epidemiological studies specifically focusing on the CKD population on conservative treatment (therefore, without AVF) are needed to assess the possible influence of PH on clinical outcomes independently of other concomitant cardiovascular or pulmonary diseases. No less important, well designed intervention studies in both pre-dialysis and dialysis cohorts are required to definitively assess if PH in CKD represents a modifiable risk factor.
Treatment of PH in CKD Patients
Current management and future therapeutic approaches for treating PH in the general population have extensively been reviewed elsewhere [62]. In the general population, treatment of PH varies according to the nature/etiology of this pathological condition (arterial, venous, hypoxic, thromboembolic, miscellaneous…). In PH secondary to congestive heart failure, treatments aiming at optimizing left ventricular function and alleviating fluid overload (e.g. diuretics, beta blockers, ACE inhibitors..) may ameliorate pulmonary circulation. The use of vasoactive agents including prostanoids, phosphodiesterase inhibitors or endothelin antagonists is usually limited to patients with established pulmonary arterial hypertension (PAH, WHO class I). Since LV disorders are highly prevalent in the CKD population the majority of CKD patients with PH in can be classified as WHO class II. No solid evidence on the treatment of PH in patients with CKD is available so far. Therefore, the recommendations for the treatment of PH WHO II category in the general population [63] can reasonably be extended also to CKD patients. The amelioration of underlying LV dysfunction appears of foremost importance in CKD stage-5 ND and in dialysis patients. As alluded to before, sleep apnea, which is highly prevalent among CKD stage-5 ND and dialysis patients, may elicit PH. Sleep apnea is currently ascribed in large part to rostral edema (edema in the hypopharynx). Rostral edema aggravates with supine position and with hypopharynx relaxation during nocturnal sleep [64]. Reduction or correction of volume excess by hemodialysis treatment intensification [65] or by peritoneal dialysis [66] produces a dramatic improvement in sleep apnea. Although there is still no proof that in dialysis patients this translates into a meaningful reduction in PASP, sleep breathing disorders should be systematically suspected and investigated in CKD patients with high PASP. Furthermore, on the basis of several observations in patients with other forms of sleep apnea, a beneficial effect on PH seems likely in dialysis patients. A randomized trial testing the vasodilator agent epoprostenol in 471 patients with PH and severe LV dysfunction was terminated ahead of time due to an increase in mortality in the treatment arm [67]. Therefore, vasodilator therapy, currently suggested for patients with established pre-capillary PH (WHO-I) should be avoided in CKD patients with PH secondary to LV disorders because potentially harmful while in CKD patients with WHO Group I PAH, these therapies should be considered on the basis of the individual risk benefit profile. In dialysis patients with persisting PH after correction of volume overload and adequate treatment of LV dysfunction, direct PH measurement by right heart catheterization and pulmonary wedge pressure evaluation might reveal whether the clinical assessment is accurate and can be helpful in discriminating whether further treatment for volume overload and/or LV dysfunction is needed. In few cases a direct treatment targeting PAH might even be indicated. Finally, interventions aimed at reducing artero-venous flow may be considered whenever clinically indicated in patients with PH and high AVF flow.
Conclusions
PH is highly prevalent in CKD patients, particularly in stage 5 patients on chronic replacement therapy by hemodialysis (Tables 14.2 and 14.3). Apart from the presence of other co-morbidities or systemic diseases, several CKD-specific risk factors including the presence of artero-venous fistula, fluid overload, sleep breathing disorders and the exposure to dialysis membranes can be implicated at various level in the genesis of CKD. PH in CKD is a potentially reversible process because, along with associated LV disorders, it may regress after kidney transplantation. However, in dialysis patients with established PH the excess risk for death may persist after kidney transplantation. PH was associated with a higher risk of death in small cohort studies conducted on stage 5 CKD pre-dialysis and dialysis patients. Large prospective studies adopting well standardized criteria of PAP measurement, such as right heart catheterization, are needed to clarify the risk of PH in CKD stage-5 ND and in dialysis patients.
Table 14.2
Summary of main studies available on PH in pre-dialysis CKD
Pulmonary hypertension in pre-dialysis | |||||
|---|---|---|---|---|---|
Study, year | Country | Patients | PH diagnostic criteria | PH prevalence (%) | Findings |
Pabst et al. [16], 2012 | Germany | 31 | mPASP ≥25 mmHg | 71 | Evidence of pre-capillary PH in 6 % of patients and post-capillary in 71 % |
Yigla et al. [17], 2009 | Israel | 127 | ePASP ≥45 mmHg | 13.4 | PH before HD initiation was associated with a higher risk of death (HR 3.6) |
Issa et al. [19], 2008 | US | 215 < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||