Pulmonary Embolism
Horiana Grosu
Ruth Minkin
Pulmonary embolism (PE) is a relatively common disease, with an estimated annual incidence of 23 cases diagnosed per 100,000 persons in the United States.1 More than 50% of cases are undiagnosed. Untreated PE has a high mortality, although risk for death is reduced significantly with anticoagulation.2
PE refers to obstruction of the pulmonary artery or one of its branches by material (e.g., thrombus, tumor, air, or fat) that originated elsewhere in the body. In this chapter we will discuss PE resulting from thrombus.
PATHOPHYSIOLOGY
Although venous thromboembolism (VTE) is a common disease, underlying pathogenic mechanisms are only partially known, particularly in comparison with those of atherothrombosis. During the past decades, progress was made in the identification and characterization of the cellular and molecular mechanisms that interdependently influence Virchow’s triad. It is now accepted that the combination of stasis and hypercoagulability, much more than endothelial damage and activation, are crucial for the occurrence of venous thrombosis; venous thrombi are mainly constituted by fibrin and red blood cell, and less by platelets.3 Most PEs originate from the deep venous system of lower extremities, iliofemoral veins being the source of most clinically significant PEs; however, upper extremities, pelvic and renal veins, and the right heart, could potentially be the embolic source as well.
After reaching the pulmonary circulation, large thrombi may lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise. Smaller thrombi continue traveling distally and are more likely to produce pleuritic chest pain, presumably by initiating an inflammatory response adjacent to the parietal pleura. Only about 10% of emboli cause pulmonary infarction, usually in patients with preexisting cardiopulmonary disease.
PREDISPOSING FACTORS
Most patients with acute PE have an identifiable risk factor at the time of presentation.4 Immobilization of only 1 or 2 days may predispose to PE.5 Among patients in whom immobilization was a predisposing factor, 65% were immobilized for more than 2 weeks.6 Other predisposing factors include surgery within the last 3 months, stroke, paresis or paralysis, history of VTE, malignancy, and central venous instrumentation within the last 3 months.4,5 Additional risk factors identified in women include obesity (BMI ≥29 kg per m2), heavy cigarette smoking (>25 cigarettes per day), and hypertension.7 The risk for PE in patients hospitalized with heart failure is twice that of hospitalized patients who do not have heart failure, and PE is a frequent cause of death in patients hospitalized with heart disease.8,9 The lower the ejection fraction, the greater the risk for VTE.10
Based on the clinical presentation, PE can be classified as massive or submassive.
Massive PE causes hypotension, defined as a systolic blood pressure <90 mm Hg or a drop in systolic blood pressure of ≥40 mm Hg from baseline for a period >15 minutes. It is a catastrophic entity that frequently results in acute right ventricular (RV) failure and death. Hypotension results from reduction in cardiac output (CO) owing to increased pulmonary vascular resistance (PVR). PVR is increased from physical obstruction of the vascular bed with thrombus and vasoconstriction, the latter due to the effects of inflammatory mediators and hypoxia. When obstruction of the vascular bed approaches 75%, the right ventricle must generate a systolic pressure in excess of 50 mm Hg and a mean pulmonary artery pressure approximating 40 mm Hg to preserve pulmonary perfusion.11 The normal right ventricle is unable to accomplish this and may eventually fail. Patients with underlying cardiopulmonary disease experience more substantial deterioration in CO than normal individuals.
When death occurs, it is often within 1 to 2 hours of the event, although patients remain at risk for 24 to 72 hours. All acute PEs not meeting the definition of massive PE are considered submassive PE.
A saddle PE is a PE that lodges at the bifurcation of the main pulmonary artery into the right and left pulmonary arteries. Most saddle PEs are submassive. In a retrospective study of 546 consecutive patients with PE, 14 (2.6%) had a saddle PE. Only two of the patients with saddle PE had hypotension.12
DIAGNOSIS
SYMPTOMS/SIGNS
The clinical diagnosis of PE is difficult because symptoms and signs are very nonspecific and occur with similar frequency in patients with and without PE.
In the prospective investigation of pulmonary embolism diagnosis II (PIOPED II), the symptoms and signs were analyzed among patients with PE who did not have preexisting cardiopulmonary disease.4 Dyspnea at rest or with exertion was most common but not a universal finding, occurring in 73% of patients with PE. The onset of dyspnea occurred over seconds or minutes in 72% of patients. The other common symptoms reported were pleuritic chest pain (44%), calf or thigh pain (44%), calf or thigh swelling (41%), cough (34%), and wheezing (21%).4
Tachypnea (respiratory rate >20 breaths per minute) occurred in 54% to 70% of patients who did not have previous cardiopulmonary disease. Tachycardia (heart rate >100 beats per minute) occurred less frequently (24% to 30%). One of the signs of right atrial, RV, or pulmonary artery pressure elevation (neck vein distension, RV lift, accentuated pulmonary component of the second sound) was documented in only 21% of patients who did not have previous cardiopulmonary disease. Lung examination showed abnormalities in 30% of patients with PE. Rales and decreased breath sounds were the most frequently detected abnormalities. Fever can be the presenting physical finding in patients with VTE, occurring with similar frequency in those with PE and pulmonary hemorrhage or infarction, and those with PE who did not have the latter. The fever was usually of low grade.13
DIAGNOSTIC TESTS
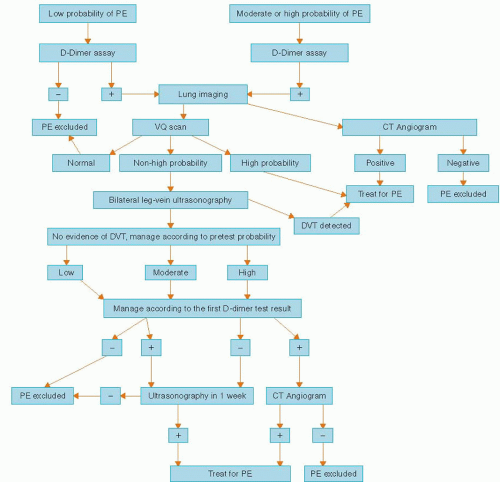
Because the clinical signs and symptoms of PE are not specific, timely diagnostic testing must be done to confirm the diagnosis (Figure 28.1).
LABORATORY TESTING
Most of routine laboratory tests are nonspecific in the setting of PE. Despite the fact that dyspnea is the main presenting symptom of PE, evaluation of oxygenation by pulse oximetry and arterial blood gas determination has a limited role in establishing the diagnosis.14
Arterial blood gas determination usually reveals hypoxemia, hypocapnia, and respiratory alkalosis, but these findings are not always seen. As an example, massive PE with hypotension and respiratory collapse can cause hypercapnia and a combined respiratory and metabolic acidosis (the latter because of lactic acidosis).
In PE, the fibrinolytic system is activated. D Dimer is a crosslinked fibrin degradation product that may be used as a marker for ongoing fibrinolysis.15 Quantitative and semi-quantitative assays for D dimer are used. For quantitative assays, a level >500 ng per ml is considered to be abnormal.16 The D-dimer level also increases with a number of conditions other than VTE15 (Table 28.1). Numerous studies have reported on utility of D-dimer assays for the diagnosis of PE. The general consensus is that these are best characterized as having good sensitivity
and negative predictive value but poor specificity and poor positive predictive value. In patients with suspected PE, D dimer is elevated in approximately 95% of cases when measured by enzyme-linked immunosorbent assay (ELISA), quantitative rapid ELISA, or semi-quantitative ELISA.16 D-dimer levels are normal in 40% to 68% of patients without PE, regardless of the assay used owing to high frequency of the positive D-dimer levels in patients without thromboembolic disease.16 The specificity decreases even further in patients with renal disease and/or increased age.17
and negative predictive value but poor specificity and poor positive predictive value. In patients with suspected PE, D dimer is elevated in approximately 95% of cases when measured by enzyme-linked immunosorbent assay (ELISA), quantitative rapid ELISA, or semi-quantitative ELISA.16 D-dimer levels are normal in 40% to 68% of patients without PE, regardless of the assay used owing to high frequency of the positive D-dimer levels in patients without thromboembolic disease.16 The specificity decreases even further in patients with renal disease and/or increased age.17
TABLE 28.1 Disorders Associated with Increased Plasma Levels of Fibrin D-Dimer | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
The ability of a normal or negative D-dimer assay to exclude acute PE depends on both the type of D-dimer assay and the clinical pretest probability for acute PE. In patients with a normal D-dimer concentration independent of the clinical probability of PE, the 3-month VTE risk is 2.3%; whereas, in patients with a high clinical probability of PE despite a normal D-dimer concentration, approximately 1 in 10 patients will still have PE.18,19 In patients with an unlikely clinical probability and a normal D-dimer concentration the risk of VTE, if the patient is untreated, is approximately 1%.19
In conclusion, the evidence indicates that a D-dimer level <500 ng per ml by quantitative ELISA or semi-quantitative latex agglutination is sufficient to exclude PE in patients with a low or moderate pretest probability of PE.
Troponin. Serum troponin I and T are frequently elevated in patients with PE, mainly in those with moderate-to-extensive clot burden, which leads to acute right heart pressure overload.20 Most studies indicate that troponins are not useful for the establishment of PE diagnosis but are a valuable tool in the prediction of adverse outcomes for patients with PE.21,22,23 and 24
An elevated brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) can predict RV dysfunction and mortality related to a PE25,26; however, they have limited usefulness as a diagnostic test for PE having sensitivity and specificity of 60% and 62%, respectively.27 Some reports suggested that BNP and NT-proBNP have a very high (approaching 100%) negative predictive value with regard to early death, which makes them particularly useful for ruling out massive life-threatening PE.28,29,30 and 31
Electrocardiography. ECG abnormalities are common in patients with PE; however, the ECG is of limited diagnostic value32 with the majority of patients presenting with tachycardia and nonspecific ST-segment and T-wave changes.33
The “classic” ECG abnormalities suggestive of PE (S1Q3T3 pattern, RV strain, and new incomplete right bundle branch block [RBBB]) are infrequent during acute PE seen mostly in patients with massive acute PE and cor pulmonale.34
ECG changes that have been reported to correlate with poor prognosis include atrial arrhythmias, RBBB, inferior Q-waves, precordial T-wave inversion, and ST-segment changes.35,36
Echocardiography. Echocardiographic abnormalities are seen in less than half of the patients presenting with acute PE. The most commonly reported findings include increased RV size, decreased RV function, and tricuspid regurgitation.37,38 These changes are more common in patients with massive PE, and the echocardiography is often used to guide the use of thrombolytic therapy.39
Pulmonary angiogram had been the gold standard for diagnosis, but now CT angiogram is effectively the diagnostic test. A negative pulmonary angiogram excludes the diagnosis of clinically significant PE. Although largely a safe procedure it still carries <2% mortality and approximately 5% morbidity in patients without significant pulmonary hypertension and hemodynamic instability.40,41 Consequently, alternative, less-invasive testing modalities have been extensively studied in patients with suspected PE.
Ventilation-Perfusion (V/Q) scan is one of the most commonly used tests for PE diagnosis. The PIOPED42 study evaluated the accuracy of V/Q scanning by comparison with the reference standard, the pulmonary angiogram.
Diagnostic accuracy was greatest when the V/Q scan was combined with pretest clinical probability. The most useful “scenarios” include patients with high clinical probability of PE and high-probability V/Q scan (likelihood of PE 95%), low clinical probability of PE and low-probability V/Q scan (likelihood of PE 4%), and a normal VQ scan that essentially excluded diagnosis of PE. However, most V/Q scans did not fall into these categories, with a diagnostic accuracy ranging from 15% to 86% that was unacceptable to either confirm or rule out the diagnosis of PE.
CT pulmonary angiogram (CT-PA) is currently the initial diagnostic test for PE in the majority of cases. In addition to greater diagnostic yield compared with V/Q scanning, this imaging may also provide an alternative diagnosis in the absence of PE that could explain patients’ symptoms.43
PIOPED II study is the largest to date assessing the accuracy of CT-PA in diagnosing PE.44 Among 824 patients with suspected diagnosis of PE, the sensitivity of CTA was 83% and the specificity was 96%.
In patients with a positive CT-PA and a high, intermediate, or low pretest clinical probability the likelihood of PE was 96%, 92%, and 58%, respectively (i.e., positive predictive value). The likelihood that PE was absent in patients with a negative CT-PA and a low, intermediate, or high clinical probability was 96%, 89%, and 60%, respectively (i.e., negative predictive value). This study suggested that CT-PA requires
concomitant pretest clinical probability assessment to be an effective diagnostic tool for confirming or excluding PE. Authors calculated pretest clinical probability using the Wells’ Criteria45 (Table 28.2).
concomitant pretest clinical probability assessment to be an effective diagnostic tool for confirming or excluding PE. Authors calculated pretest clinical probability using the Wells’ Criteria45 (Table 28.2).
The study investigators concluded that the predictive value CTA is high with a concordant clinical assessment, but additional testing is necessary when the clinical probability is inconsistent with the imaging results. Negative CT-PA has excellent negative predictive value for excluding PE. Patients with negative CT-PA combined with negative lower extremity Doppler, and low or intermediate clinical probability have <2% incidence of PE within 3 months follow up.46
Numerous studies have evaluated the safety and efficiency of relatively complex combinations of clinical decision rules and diagnostic tests in patients with suspected PE to develop an effective algorithm for evaluation of patients with suspected acute PE.46,47 and 48 The Christopher Study48




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree