Chapter 8 Pulmonary circulation
After reading this chapter, you should:
The pulmonary circulation is contained wholly within the thorax. It is, therefore, much shorter than most regional beds of the systemic circulation. As well, all segments of the pulmonary vessels are slightly larger in radius than the corresponding segments of the systemic vasculature. The net result of these two factors is that the pulmonary circulation exerts a resistance to flow only around 15% of that in the systemic circuit. Hence it requires a correspondingly far lower pressure gradient in order to move the same cardiac output. At rest, pulmonary arterial pressure is typically 25/8 mmHg, giving a mean arterial pressure of the order of 14 mmHg, and mean pulmonary capillary pressure is 7–8 mmHg rather than the 25–30 mmHg seen in systemic vascular beds.
FUNCTIONAL CONSEQUENCES OF LOW PULMONARY BLOOD PRESSURE
Gas diffusion
In systemic capillaries the balance between oncotic and hydrostatic pressures mean that small increases in hydrostatic pressure caused, for example, by reduced arteriolar resistance will result in significant movement of plasma water into the interstitium. When a large tissue mass, such as skeletal muscle, is involved, this movement will cause a substantial reduction of plasma volume (see Chapter 9, p. 112), but the increased interstitial volume does not prejudice diffusion of solutes between cells and bloodstream because the intercellular connective tissue minimizes tissue expansion. In the lung, by contrast, there is little supporting tissue. If water moves into the interstitial space separating the pulmonary capillaries from the alveoli, it pushes these two structures further apart and increases the distance over which gases must diffuse between air and plasma.
The practising exercise physiologist is most likely to encounter pulmonary oedema as a consequence of high altitude, since it occurs in many healthy individuals who ascend to heights greater than 3000 m, well below the altitude of many ski resorts and permanent settlements. We will return to examine the reasons for this response to altitude in Chapter 12 (p. 151).
Right ventricular function
Left ventricular perfusion occurs only during diastole because during systole the left coronary vessels are compressed by the surrounding cardiac muscle (see Chapter 2, p. 6). By contrast, since right ventricular pressure does not normally rise above 25 mmHg, the coronary vasculature supplying the right ventricle is not compressed at any stage of the cardiac cycle and coronary flow is continuous. The increased efficiency of coronary oxygen delivery is reflected in a lower density of coronary blood vessels in the right heart. However, this design feature has potentially disadvantageous results if left atrial pressure becomes chronically elevated and right ventricular pressure rises in response to this higher afterload.
Under these circumstances, right coronary perfusion during systole becomes progressively reduced and the metabolic demands of the right-side cardiac muscle cells are less efficiently serviced. Over time, the right ventricle will respond to the high afterload by muscle hypertrophy. This will exaggerate the coronary insufficiency, both by providing greater metabolic demand for oxygen delivery and by producing more coronary compression. As a result, chronic hypoxic damage to the muscle of the right heart, with an inability to increase contraction appropriately in response to increased filling (right cardiac failure) is a common long-term effect of elevated pulmonary blood pressure (see Chapter 12, p. 148).
REGIONAL MATCHING OF VENTILATION AND PERFUSION
Vertical variations
Although both ventilation and perfusion rise towards the base of the lungs, the gravitational effect on regional blood flow is rather more powerful than the vertical variation in ventilation, with the result that the ratio of ventilation  to perfusion
to perfusion  is around 3 at the apices and around 0.5 at the bases. Exact matching of the two is achieved only over a relatively narrow region of lung corresponding to about the mid-sternal level (Fig. 8.1).
is around 3 at the apices and around 0.5 at the bases. Exact matching of the two is achieved only over a relatively narrow region of lung corresponding to about the mid-sternal level (Fig. 8.1).
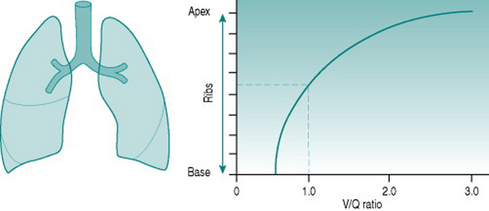
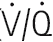 ) ratio variation in the vertical lung. At rest, optimal matching (
) ratio variation in the vertical lung. At rest, optimal matching (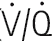 = 1.0) occurs only over a relatively narrow region around the mid-sternal area.
= 1.0) occurs only over a relatively narrow region around the mid-sternal area.


