Fig. 19.1
Angiographic morphology of the RV in PAIVS. (a) Mild RV cavitary hypoplasia with well-developed infundibulum, inlet and apical parts and ‘tripartite’ RV. There is moderate TR and the RA is dilated. (b) In the ‘bipartite’ RV, the inlet and infundibulum are well developed, but the apical part is almost obliterated by muscles. There is also moderate TR. (c) PAIVS with severe TR from a dysplastic tricuspid valve. Markedly dilated and thin-walled RV and RA. (d) Severe hypoplasia of the RV cavity with only the inlet part present, i.e. ‘unipartite’. RV-coronary connections to both the left and right coronary systems. Reflux of contrast into the aortic root (arrow). No TR (RA right atrium, RV right ventricle, PAIVS pulmonary atresia with intact ventricular septum, TR tricuspid regurgitation)
19.2.1 Major Anatomic Subtypes
(i)
Imperforate membranous valve
In this group the valve leaflets are completely fused, leaving an imperforate membrane. The infundibulum is usually well developed and smooth walled, though occasionally abnormal muscle bundles may cause additional, fixed subvalve stenosis. The RV cavitary hypoplasia is often of mild to moderate degree, either with all three parts (inlet, infundibulum and apical) present, i.e. ‘tripartite’, or quite commonly the RV is ‘bipartite’ where there is near obliteration of the apical part by muscles, while the remaining two are well developed (Fig. 19.1a, b). The atretic membranous valve usually is a thin membrane which has a mild or moderately hypoplastic annulus. In contrast the pulmonary artery root and its sinuses are usually well developed, seen angiographically ‘cupping’ over the membranous valve and infundibulum (Fig. 19.2a, b). The main pulmonary artery and its branches are also well developed. Occasionally, the valve is thick and immobile with the pulmonary artery root and sinuses being poorly developed (Fig. 19.2c, d). In a small minority, the RVOT is markedly dilated such that the usual tunnel-like configuration is lost with the small valve and small annulus facing the dome-like infundibulum (Fig. 19.2d). This is usually seen in association with severe Ebstein’s malformation of the tricuspid valve.
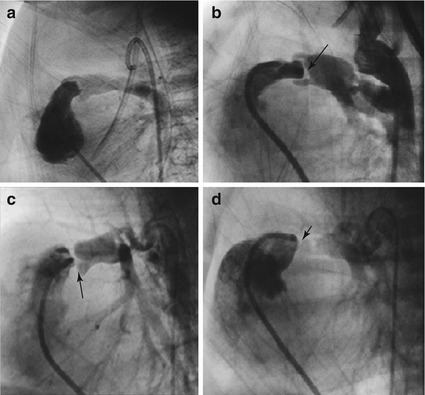

Fig. 19.2
The RVOT valve and pulmonary artery. Simultaneous angiograms in the RVOT and descending aorta opposite the PDA are performed to outline these structures. (a) Well-developed infundibulum, thin membranous valve and mildly hypoplastic annulus. The main pulmonary artery and sinuses are well developed, ‘cupping’ over the valve membrane and annulus. (b) Similar anatomy as in (a) but the valve membrane is ‘slightly’ thicker (arrow). (c) Very thickened valve plate (arrow). The sinuses of the pulmonary artery root are less well developed. (d) Markedly dilated RVOT in a patient with severe TR from associated Ebstein’s malformation of the TV. The pulmonary annulus is small. The pulmonary artery root and sinuses are poorly developed (arrow) (RVOT right ventricular outflow tract, PDA patent ductus arteriosus, TR tricuspid regurgitation, TV tricuspid valve)
The presence of RV-coronary connections is less common in this group.
The morphologic subtype illustrated by Fig. 19.1a, b is suited for valve perforation and balloon dilation, having the best chance of achieving 2-ventricle or 1½-ventricle circulation. It will be the focus of this chapter.
(ii)
Muscular atresia
Patients with complete or near-complete muscular atresia of the infundibulum generally have severely hypoplastic RV cavity where only a small inlet is present (‘unipartite’), the rest obliterated by muscles. The RV is often markedly hypertensive, and RV-coronary connections are commonly present (Fig. 19.1d). The coronary circulation is RV dependent because of atresia or severe stenoses of the proximal coronary arteries and has been described in anywhere from 3–34 % of these patients. This subgroup of PAIVS is managed as hearts with single-ventricle circulation as the RV is too small to function as an effective pulmonary pump.
The tricuspid valve is not uncommonly dysplastic with varying degrees of regurgitation. Severe Ebstein’s malformation with markedly dilated, thin-walled right atrium (RA) and RV is a well-recognized association.
The PDA in PAIVS tends to resemble that of isolated PDA, arising from the distal arch and inserting onto the dome of the main pulmonary artery (MPA) away from the orifice of the proximal left pulmonary artery (LPA). Though they are usually elongated, they are seldom tortuous. However, as in other cyanotic heart disease, the PDA may have a more complex morphology and insert onto the proximal LPA causing stenosis of this branch.
19.3 Pathophysiology and Clinical Presentation
Because of complete RV outflow obstruction and major aortopulmonary collateral vessels being rarely present, the pulmonary circulation is duct dependent. There is an obligatory right-to-left shunt through the patent foramen ovale (PFO); hence, cyanosis at birth – linked temporally to ductal constriction – is the most common presentation. However in the current era, diagnosis is often made antenatally during fetal cardiac screening. As in lesions with duct-dependent pulmonary blood flow, severe cyanosis, acidosis and circulatory collapse may occur with rapid closure of the PDA. The RV is markedly hypertensive especially when the tricuspid valve is competent. Marked RV hypertrophy and small RV cavity cause reduced RV compliance and may lead to persistence of right-to-left shunt even after RV pressure is normalized following balloon dilation. Severe tricuspid regurgitation may cause marked enlargement of the right atrium. In the presence of RV-dependent coronary circulation, ischaemia/infarction may occur when the RV is decompressed following pulmonary valvotomy (surgery) or balloon dilation.
19.4 Treatment Options, Indications and Patient Selection
Management strategy should be formulated according to the likelihood of 2-ventricle (or 1½-ventricle) circulation vs single-ventricle physiology, and this is based on the RV anatomy at presentation.
Patients with definite muscular atresia of the infundibulum and diminutive RV should be directed towards the Fontan track at the outset, a largely surgery-based management beginning with the BT shunt at diagnosis. Catheter interventional therapy is limited to PDA stenting as alternative to surgical shunt and balloon atrial septostomy.
In patients with well-developed infundibulum where the atresia is limited to a membranous imperforate valve, the goal is to establish unobstructed antegrade flow into the pulmonary vascular bed, abolish RV hypertension, reduce tricuspid regurgitation, promote RV growth and in the occasional patients disrupt RV-coronary connections and restore normal coronary perfusion. The final objective is to achieve biventricular circulation or at least 1½-ventricle circulation in those whose RV fails to grow adequately. This is conventionally managed with closed surgical valvotomy or an open procedure with RVOT reconstruction. Many surgeons electively perform concomitant BT shunt as a significant number remain cyanotic after a successful valvotomy procedure.
Today catheter intervention with radiofrequency-assisted valvotomy and balloon dilation (RFV-BD) is the preferred method of opening the valvar atresia and establishing antegrade flow to the pulmonary arteries. PDA stenting may be performed at the same time in patients whose RV is deemed small and who likely require augmentation of the pulmonary blood flow following RFV-BD. The assignment of individual patients towards the single-ventricle vs 2-ventricle track is based on the initial echocardiographic assessment and cardiac catheterization.
19.5 Pre-procedure Imaging
Echocardiography provides detailed information for the initial planning of management. An important parameter in echocardiographic assessment of PAIVS is the size and morphology of the RV, i.e. whether the patient is a likely candidate for RFV-BD, i.e. a membranous atresia with well-developed infundibulum and at most moderate RV hypoplasia (tripartite or bipartite RV), or one destined for the Fontan track, i.e. muscular atresia of the infundibulum and a diminutive, unipartite RV.
The measurement of TV Z-score and TV/MV annulus ratio provides a semiquantitative measure of RV size. Doppler echocardiography provides additional information with the degree of tricuspid regurgitation and an estimate of the RV systolic pressure.
Finally, branch pulmonary artery size and confluence is assessed. More importantly evaluation of the PDA morphology should be noted for purposes of PDA stenting if this forms part of the management. Large RV-coronary connections may be visible on colour Doppler, but its delineation can only be achieved with angiography.
Angiography provides a more precise information and is essential in the formulation of a management strategy. Accurate assessment of the nature of pulmonary atresia, the infundibulum, the pulmonary valve annulus and the PDA morphology is obtained as part of the interventional procedure. We recommend this to be performed in all patients, even those destined for the Fontan track particularly for a detailed evaluation of RV-coronary connections.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


