Indications and Preoperative Evaluation
Indications and Preoperative Evaluation
The indication for a sleeve resection for lung cancer is well established: A tumor arising at the origin of a lobar bronchus and/or at the origin of the lobar branches of the PA, but not infiltrating as far as to require PN (Fig. 40.1A,B,C). In addition, a sleeve resection may be indicated when N1 nodes infiltrate the bronchus and/or the PA from the outside, as is often the case in the left upper lobe tumors requiring a combined reconstruction of the bronchus and the PA. From a functional point of view, sleeve lobectomy (SL) is strictly indicated in patients who cannot withstand PN, but recent experiences have shown that the advantages of sparing lung parenchyma are evident even in patients without cardiopulmonary impairment.
It is difficult to establish the correct indication for a reconstructive procedure of the PA preoperatively. Computed tomography (CT) with contrast medium is the most used diagnostic tool. Angiography and magnetic resonance imaging (angio-MRI) of the blood vessels can provide useful information to define the pattern of infiltration, but the decision is usually made intraoperatively.
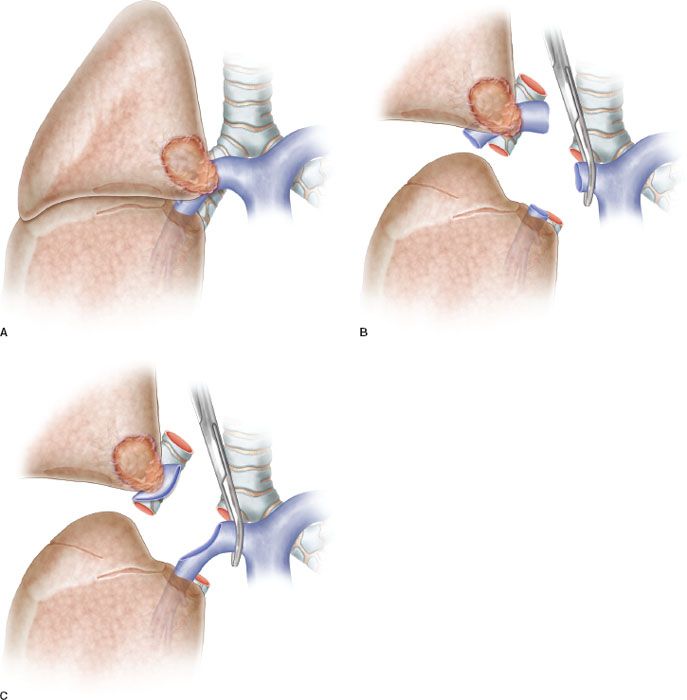
Figure 40.1 A: Partial resection of the pulmonary artery requiring a patch reconstruction. B: Hilar tumor with extensive infiltration of the pulmonary artery requiring a sleeve resection. C: Sleeve resection of the pulmonary artery requiring a reconstruction by end-to-end anastomosis.
PA infiltration degree and extension is not always clearly indicated at preoperative imaging. Sometimes discrepancies between radiologic evidences and intraoperative findings may be responsible of wrong indications, since the preoperative study may overestimate or underestimate the vascular involvement.
Oncologically, the primary goal is in every case the complete resection of the tumor with free resection margins. The decision to perform PN or SL is based on both oncologic and physiologic considerations. There is evidence that PN, especially right PN, is a disease itself, with severe impairment of lung function and quality of life after surgery, and therefore the latter intervention should be avoided whenever possible.
 SURGERY
SURGERY
The first step of the operation consists of achieving full control of the proximal portion of the PA. Although division of the superior pulmonary vein can facilitate the exposure of the PA, transection of this vessel should be postponed until the feasibility of the procedure has been ascertained.
The resection phase begins once the main and distal PA, the bronchus, and both pulmonary veins have been duly prepared. The superior pulmonary vein is generally divided first. Clamping of the proximal PA is then performed after systemic heparinization. In the past we used to clamp the inferior pulmonary vein to obtain backflow control. Actually, we prefer to clamp the PA distally to the tumor infiltration.
The dose of intravenous heparin represents the only intraoperative management modification adopted by the authors over time. We actually prefer to administer 1,500 to 2,000 units (about 25 units/kg) instead of a dose between 3,000 and 5,000 units used in the past. Heparin dose has been reduced to prevent postoperative oozing, especially from the lymphadenectomy sites. We have observed that, with progressive reduction of mean clamping time due to increased experience, this dose proves effective in avoiding the risk of thrombosis. Heparin is not reversed by protamine after declamping, once the vascular reconstruction has been completed.
Resection and Reconstruction Phase
Partial Resection and Patch Reconstruction
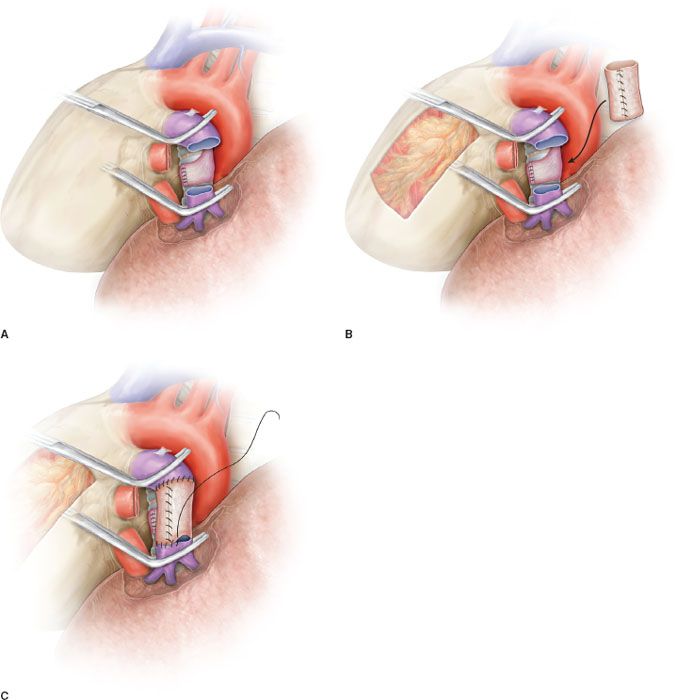
Patch reconstruction technique can be applied to a variety of conditions. These may range from limited infiltration of the origin of segmental arteries to larger resection of the PA involving less than one-half of the vessel circumference (Fig. 40.2A). In case of more extended infiltration a sleeve resection with end-to-end anastomosis or conduit interposition has to be performed (Fig. 40.1A,B,C).
Various materials have been proposed and used for patch reconstruction, including synthetic or biologic options, with the latter being preferred because of the higher biocompatibility.
One biologic option is represented by the venous patches including azygos and saphenous vein patches. Azygos vein patches present adequate characteristics, but are available only on the right side and provide limited amount of tissue. Other venous patches generally require separate surgical procedures for tissue harvesting.
Among the biologic materials, the authors recommend the use of the autologous or bovine pericardium. In particular, they prefer the autologous pericardium because it has a number of advantages: It shows adequate thickness and resistance, it is cost free and available on both sides of the chest, and it has superior biocompatibility if compared with the bovine pericardium. Moreover, it provides an amount of tissue that is sufficient also for large defect repair and does not require a separate surgical procedure for its harvesting. However, fresh autologous pericardium has some technical limits, since it has a tendency to shrink and curl making more difficult the adaptation and suturing of the patch to the vascular wall.
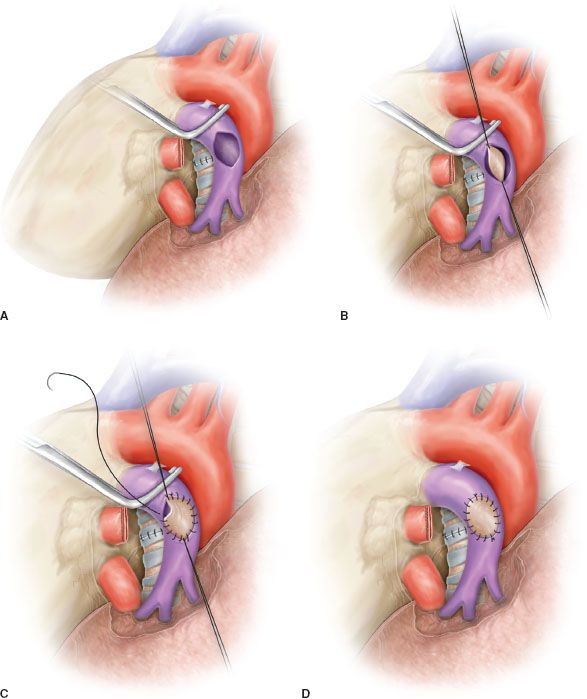
Figure 40.2 Partial resection and patch reconstruction of the pulmonary artery. A: Left pulmonary artery after partial resection of the vascular wall. An oval defect is visible before reconstruction. B: Patch reconstruction: The patch is secured to the vascular wall of the pulmonary artery by two stay sutures. C: Patch is sutured (running suture) to the vascular wall. D: Completed patch reconstruction.
Conversely, bovine pericardium displays limited elasticity and exhibits even and stiff edges that considerably reduce the pitfall of harvesting, trimming, and suturing the autologous pericardium.
To improve technical features of the autologous pericardium, over the last decade, the authors have devised and employed an intraoperative method of fixation of the patch by a glutaraldehyde-buffered solution. The glutaraldehyde preservation of the pericardium minimizes its tendency to retract and curl, thus allowing an easier vascular reconstruction, and reducing the risk of bleeding from the patch suture related to the elastic recoil of the autologous pericardium. Harvesting of the autologous pericardium is performed anteriorly to the phrenic nerve, leaving open the pericardial defect.
The patch is appropriately trimmed and secured to the arterial wall by two stay sutures at the opposite sides of the vascular defect (Fig. 40.2B). Suturing is done with 5-0 or 6-0 monofilament nonabsorbable material proceeding from top-to-bottom “artery first,” and then continuing from bottom-to-top “patch first,” while the assistant grasps and stretches the patch (Figs. 40.2C,D and 40.3). The inferior stay suture is not tied: It is used only to keep the patch in place and is removed when the suture line reaches its level.
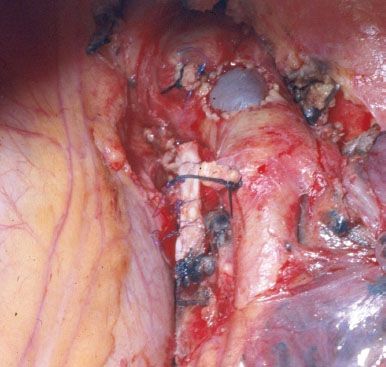
Figure 40.3 Intraoperative picture showing a completed patch reconstruction of the left pulmonary artery.
In patients requiring also a bronchial anastomosis, the PA patch reconstruction is generally performed first, to reduce clamping time.
Check of the suture line after residual lung re-expansion is mandatory, especially if fresh autologous pericardium is used for reconstruction, since retraction of the patch margins may result in bleeding sites when tension changes are applied on the PA axis.
Sleeve Resection and Reconstruction by End-to-end Anastomosis
Sleeve resection of the PA is always required when half or more of the vessel circumference is infiltrated by the tumor (Figs. 40.1B,C and 40.4A). In every case, after resection, frozen section histology should be made on vascular margins and if tumor infiltration persists, PN has to be performed without hesitation.
Transection of the artery proximally and distally to the tumor has to ensure regular and even margins to allow subsequent proper placement of sutures. In addition, regular borders facilitate, during reconstruction, the correction of the caliber discrepancy that is usually found between the two vascular stumps after resection. Due to the elasticity of the arterial wall, caliber discrepancy never represents a technical problem.
In patients requiring a concomitant bronchial anastomosis, the PA reconstruction is generally postponed, since exposure of the bronchial stumps is better when the artery is divided. Moreover, after completion of the bronchial anastomosis the bronchial axis is shortened thus reducing tension on the vascular anastomosis. Distance between the two vascular ends can be further reduced by elevating the lower lobe while suturing. If the distance appears excessive before reconstruction, a prosthetic conduit interposition is indicated. The anastomosis is performed by running suture using 5-0 or 6-0 monofilament nonabsorbable material (Figs. 40.4B,C and 40.5).
The posterior (mediastinal) portion of the anastomosis is performed first, then the suture is completed in the anterior portion, which is the easiest part of the reconstruction. Once the anastomosis has been completed, suture is tied after removal of the distal clamp to restore backflow and to allow air drainage through the untied part of the suture line.
It is important to test the arterial axis and suture line after re-expansion of the residual lobe. Reinflation of the lower lobe elevates the hilum and can produce rotation and kinking of the PA. This may distort suture line and reopen bleeding sites that are not visible when the artery is stretched downward by the atelectatic lower lobe.
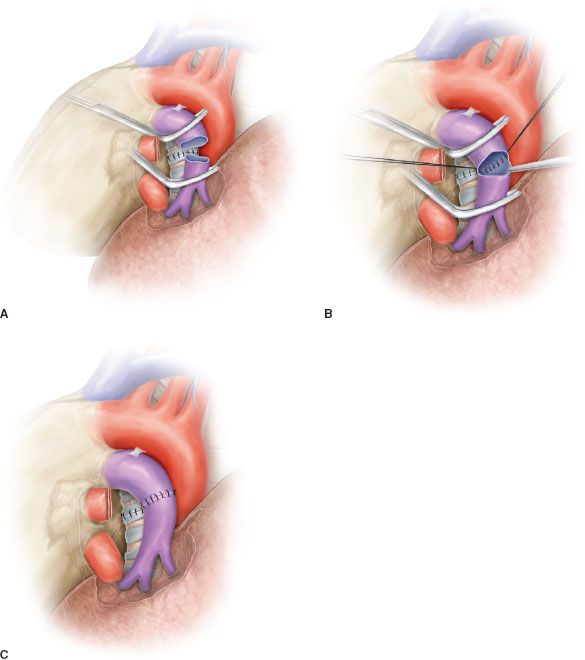
Figure 40.4 Sleeve resection and end-to-end anastomosis of the pulmonary artery. A: Sleeve resection of the left pulmonary artery. The PA is clamped proximally and distally and the two vascular stumps are visible before reconstruction. B: End-to-end anastomosis by running suture. C: Completed end-to-end anastomosis.
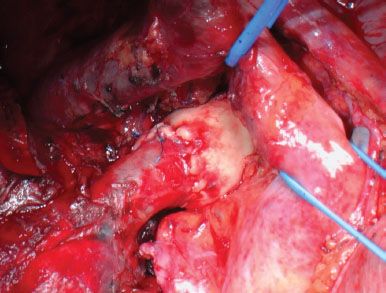
Figure 40.5 Intraoperative picture showing a completed anastomotic reconstruction of the right pulmonary artery.
Sleeve Resection and Reconstruction by a Prosthetic Conduit
In some patients after sleeve resection of the PA an excessive distance between the two vascular stumps may result. This condition could produce a high tension on the anastomosis. Such technical situation may occur, usually on the left side, in those cases requiring resection of a long segment of the PA without associated bronchial sleeve resection, because the lobar bronchus is not involved. In these cases the vascular reconstruction cannot be performed by a direct end-to-end anastomosis and a prosthetic conduit interposition is required (Fig. 40.6A,B).
Although the need for a vascular conduit is not a frequent condition, various materials and different techniques have been proposed for such reconstructive procedure.
Biologic materials are generally preferred because of higher biocompatibility and lower risk of thrombosis. The authors have reported the successful use of the autologous and the bovine pericardium. Intraoperatively, the pericardial leaflet is trimmed to a rectangular shape and wrapped around a chest tube or a syringe of appropriate diameter and sutured longitudinally (Figs. 40.6B and 40.7). In our initial experience this suture was performed manually with a 6-0 monofilament nonabsorbable material (Fig. 40.7). More recently we have described a technical alternative with a mechanical suture using a linear stapler for the conduit construction. The creation of a 1 to 2 cm conduit is so accomplished. When the autologous pericardium is employed the epicardial surface is oriented inside the conduit lumen.