1. Congenital vascular malformations:
Unilateral absence of a pulmonary artery, atresia, pulmonary artery stenosis associated with extreme tetralogy of Fallot or anomalies in the intraparenchymal vessels, due to defects in the media or to misalignment of the pulmonary vessels (Fig. 2.1)
Pulmonary artery aneurysm associated with mycosis, infections such as syphilis, vasculitis (Behçet’s disease), pulmonary hypertension, trauma or congenital cardiac diseases (principally, patent ductus arteriosus and atrial septal defects)
2. Thrombotic pulmonary embolism
3. Non-thrombotic pulmonary embolisms, caused by fragments of bone marrow, tumours, fat, foreign bodies, air bubbles, parasites or those associated with pregnancy and puerperium
4. Thrombotic angiopathy associated with chronic haemolytic anemia in sickle cell disease
5. Pulmonary veno-occlusive disease
6. Pulmonary capillary haemangiomatosis
7. Pulmonary hypertension originating in arterial or venous vessels (pulmonary edema and chronic venous hypertension)
8. Hypoxic pulmonary hypertension, such as high-altitude cardiopulmonary diseases, sleep apnea syndrome or kyphoscoliosis
9. Structural lung diseases, infiltrative or obstructive, which secondarily affect the pulmonary circulation
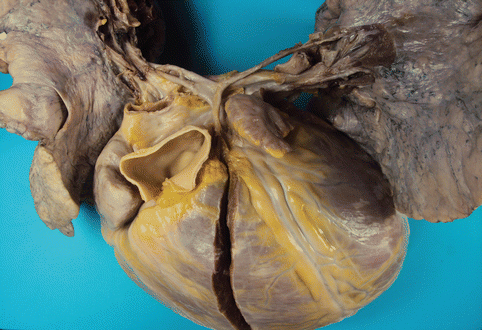
Fig. 2.1
A 33-year-old male with extreme tetralogy of Fallot: pulmonary atresia and severe hypoplasia in the pulmonary arteries, which are reduced to a fibrous cord. The patient presented with severe heart failure. Severe cardiomegalia (>1,000 g) with dilation and hypertrophy of the right cavities
For reasons of image economy, this chapter will deal only with the two most significant pathologies encountered by the pathologist during autopsy: pulmonary arterial hypertension, specifically idiopathic and familial forms, and pulmonary thromboembolic disease.
For a detailed analysis and study of additional pulmonary pathologies, please refer to the general pathology textbooks listed in the bibliography section of this chapter.
2.2 Pulmonary Thromboembolism
Pulmonary thromboembolism (PTE) and deep venous thrombosis (DVT) encompass different clinical manifestations of a single disease, venous thromboembolism (VTE). Because of its non-specific clinical manifestations, diagnosis is complex and the disease is often identified at autopsy. In autopsy series PTE constitutes the cause of death in approximately 4 % of sudden deaths that occur outside of the hospital.
The so-called Virchow’s triad of interrelated factors: vascular stasis, endothelial damage and abnormal blood flow, still constitutes the basis for understanding the pathogenesis of thrombus formation. Identified thrombogenic risk factors include: reduced mobility, old age, malignancy, recent medical or surgical disease, trauma, spinal lesion, obesity, smoking, pregnancy and puerperium, polycythemia vera, and several medications such as oral contraceptive drugs. Recently, a correlation has been established between fatal PTE and a history of psychiatric disorder, or treatment with antipsychotic drugs.
Together with these acquired factors, additional hereditary disorders have been related with a state of hypercoagulability, which facilitates the development of venous thrombosis. Included among these hereditary disorders are hereditary thrombophilias such as mutations in Factor V (Factor V Leiden), prothrombin gene mutation (20210A), antithrombin I, II and III deficiencies, Protein S, Protein C or plasminogen deficiencies, etc. Additional syndromes including antiphospholipid syndrome or hyperhomocysteinemia are also associated with hypercoagulability.
In 95 % of PTE cases, the thrombus originates in the deep venous system of the lower extremities (Fig. 2.2a–c). Different pathological conditions result in thrombus formation in the crural, femoral or iliac veins. In exceptional cases, the origin of the embolizing thrombus has been discovered in diverse venous territories such as the mesenteric veins (Fig. 2.3), the right heart (Fig. 2.4a–c) and the upper extremities. Furthermore, the presence of a central venous cathether constitutes a causal factor for thrombosis and subsequent pulmonary embolism, especially in children.
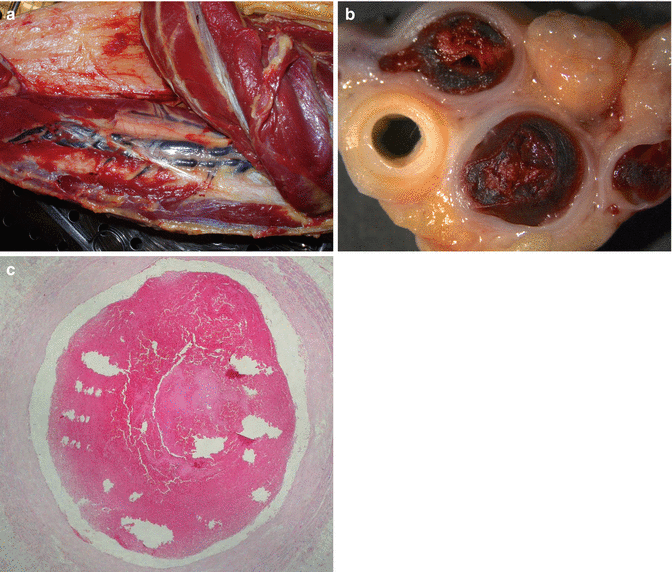
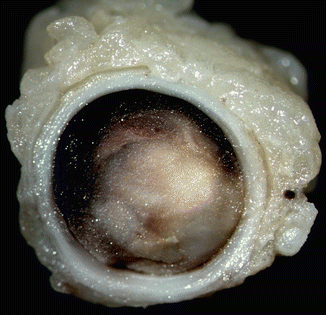
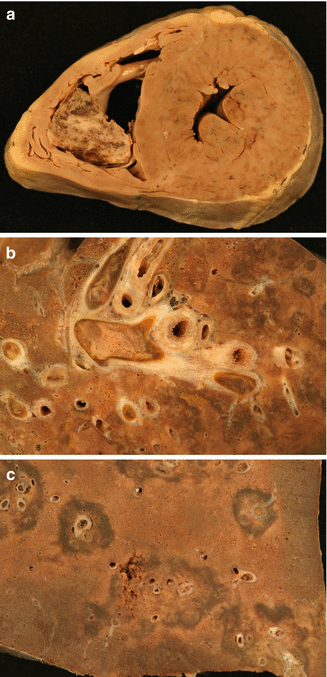

Fig. 2.2
(a) Thrombosis in the deep venous system of the leg. (b) Stereoscopic image of a transversal section of the venous thrombosis. (c) Histological image of a thrombus located in the posterior tibial vein, formed by platelets and red blood cells

Fig. 2.3
Acute thrombosis in the superior mesenteric vein in a 16-year-old male who died suddenly due to PTE. Analysis for hereditary thrombophilia was negative

Fig. 2.4
(a) Intracavitary thrombus adhered to the right ventricular endocardium, which was the cause of a masive fatal PTE in an 8-year-old boy with corticosteroid-dependent nephrotic syndrome. (b) Massive thrombosis in secondary and tertiary pulmonary arteries. (c) Necrotizing-haemorrhagic perivascular lesions. Analysis for hereditary thrombophilia indicated a heterozygous mutation in the prothrombin 20210A allele
Once the embolizing thrombus has reached the territory of the pulmonary arteries it can provoke two different effects in the pulmonary circulation: on the one hand a hypoxic state can arise since the thrombus obstructs, totally or partially, blood flow in the pulmonary respiratory units, altering the ventilation/perfusion relationship. This can lead to the release of substances that would subsequently produce pulmonary vasoconstriction, aggravating the mechanical ventilation effect. Alternatively, the flow obstruction could mediate a haemodynamic effect, resulting in acute pulmonary artery hypertension (PAH). In turn, this acute state of PAH produces right ventricular pressure overload that ultimately causes right cardiac failure.
The clinical severity of PTE depends on factors such as the size of the embolus (from lodged thombi that affect both pulmonary arteries at the proximal level, to multiple disseminated microthrombi), which determines the number of obstructed vessels and the relative percentage of the affected pulmonary artery bed, the speed of establisment of an acute PAH, and the previous state of the pulmonary circulation. Thus, pre-existence of pulmonary or cardiac pathologies determines whether or not the double circulation through the bronchial arteries can counteract the damage caused by obstruction in the main pulmonary arteries. The existence of this supplementary circulation, and the speed at which the effects of PAH appear, especially in cases of massive PTE, explains the relatively low incidence of pulmonary infarctions found at autopsy series following a PTE episode. This ranges from 6 to 20 % depending on the autopsy series, and can be even lower in cases of sudden death with non-organised thromboemboli.
Clinical diagnosis is complex. Patients with DVT in the lower extremities can be asymptomatic or can present with pain, heat or inflammation. Pulmonary embolism must always be considered in cases of unexplained dyspnea. The majority of pulmonary emboli are multiple and affect the inferior lobes. In the case of small emboli that do not cause significant obstruction in the pulmonary circulation, the clinical symptoms can manifest as acute pleuritic chest pain, or as haemoptysis. Only 10 % of emboli cause pulmonary infarcts, usually in patients with pre-existing cardiopulmonary pathology. In the most severe cases, the clinical picture can be syncope and hypotension. Symptoms can be more subtle in older patients, where it is not unexpected to find that the main clinical manifestation is syncope. This contrasts with younger patients, where the clinical presentation is more florid. In up to 25 % of cases DVT can present as sudden death.
Imaging techniques are fundamental for the diagnosis of PTE. The most commonly-used are ventilation/perfusion gammagraphy and angio-TC. The choice between one technique and the other depends on its availability, and the experience of the centre. Magnetic resonance imaging and pulmonary arteriography are techniques that are not usually available in an emergency setting. Doppler echography is the method of choice to diagnose the presence of DVT. Additional tests including ECG, echocardiogram, dimer-D test, troponin and natriuretic peptide determination, can be helpful in the diagnosis of PTE and have independent prognostic value.
When death occurs, the most significant pathological findings are discovered in the pulmonary arteries, pulmonary tissue, and the heart. With PTE, the autopsy must be exceptionally methodical in order to locate the thrombogenic focus, usually situated in the deep veins of the lower extremities which must be systematically examined during necropsy.
Thromboemboli causing PTE are found in the pulmonary arteries. Very large thrombi tend to remain located in the main arterial trunk, causing in some cases an obstruction of both pulmonary arteries (“saddle” embolus) (Fig. 2.5a). Smaller thrombi can provoke an obstruction in the main branches of the pulmonary artery (Figs. 2.6 and 2.7), while the smallest thrombi obstruct the intrapulmonary branches.
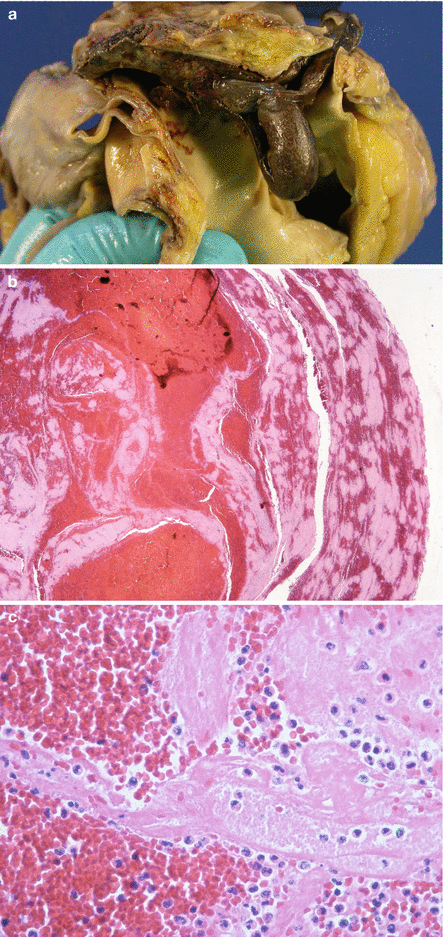
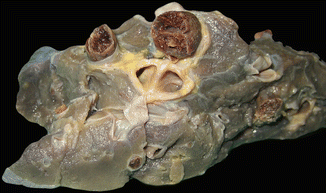
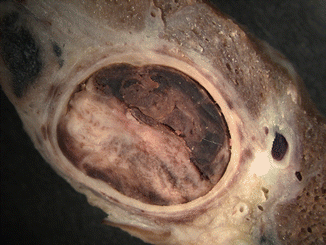

Fig. 2.5
Sudden death in a 39-year-old pregnant woman. (a) Thrombus lodged at the bifurcation of the pulmonary artery (“saddle” embolus). (b) Microscopic image of the thrombus where the Zahn lines are evident. (c) Microscopic detail of the thrombus with abundant platelets

Fig. 2.6
Thromboemboli in the main pulmonary arteries. Image resembling “toothpaste squeezed from a tube”

Fig. 2.7
Detailed image of a red thromboembolus occluding a main pulmonary artery
Thrombi can be divided morphologically into two major types according to the degree of chronological evolution: grey thrombi (Fig. 2.8) and red thrombi (Figs. 2.5 and 2.6). Grey thrombi are compact, whitish in appearance, and are laminated when sectioned. Red thrombi are also compact, but are dark red in colour and display alternate white and red layers when sectioned (Zahn’s lines). Both thrombi occlude, but do not perfectly mould, the arterial lumen. The contour of the thrombi reflects the shape of the original venous mould, and even the imprinting of the cardiac valves of the right heart, through which they progress towards the pulmonary arteries. In microscopy, the Zahn’s lines correspond to alternating layers of erythrocyte aggregates and platelets mixed with fibrin (Fig. 2.5b, c). These layers, or lines, reflect the mechanism through which thrombi are produced in situations of venous stasis at the thrombogenic focus and result from the progressive deposition of blood elements on a thrombus originally adhered to a damaged venous endothelium. In situations where many neutrophils can be microscopically identified in the thrombus, the term septic thrombus is given, and suggests the presence of an associated infectious process.
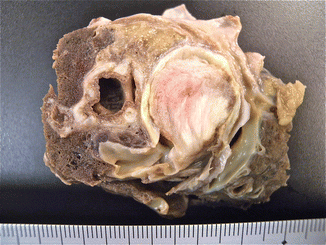

Fig. 2.8
Gray thromboembolus in the main pulmonary artery with a near-complete occlusion in the vascular lumen and a laminated surface on section
Red thrombi found in the pulmonary artery bed have to be distinguished from postmortem clots (Fig. 2.9). Table 2.2 describes the morphological differences between acute vital thrombi, responsible for PTE, and postmotem clots.

Fig. 2.9
Post-mortem blood clot. Biphasic colour can be observed with light areas clearly separated from dark areas (at the extremes of the blood clot)
Table 2.2
Macroscopic characteristics of the vital thrombi and postmortem blood clots (Groshong et al. 2008)
Acute vital thrombus | Postmortem blood clot | |
|---|---|---|
Colour | Dark red | Biphasic (red/yellow) |
Consistency | Firm | Soft and jelly-like |
Shape | Coiled | Forms branches |
Mould of the leg veins | Mould of the pulmonary artery | |
Lineal striations | Present | Absent |
Vascular distension | Present | Absent |
Adhesion to vessels | Present | Absent |
Perivascular haemorrhage | Present | Absent |
Venous thrombi, once they obstruct the pulmonary arterial lumen, undergo a remodelling process that provokes, in the first instance, the adherence of the thrombus to the arterial endothelium (Fig. 2.10). Next, the thrombus is replaced with connective scar tissue and finally, the thrombus is rechanneled with newly-formed lumen in the intially-occluded arterial lumen (Fig. 2.11). This temporal evolution can be reflected in the findings from a microscopic study of the thrombus. However, this classical scheme, which is applicable to thrombotic lesions found in peripheral veins, could be accelerated in the pulmonary arteries. Recent studies have proposed a classification system based on only three identifiable temporal phases, as summarised in Table 2.3. Several authors recommend to focus the thrombus-dating study at the origin of DVT, rather than at the pulmonary thrombi, given the more unpredictable evolution in this latter location.
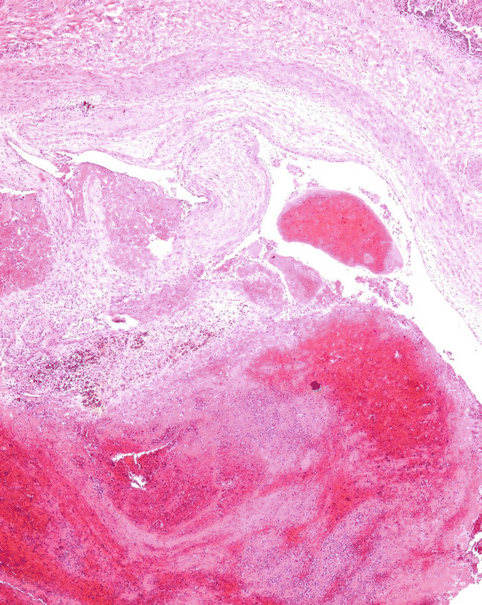
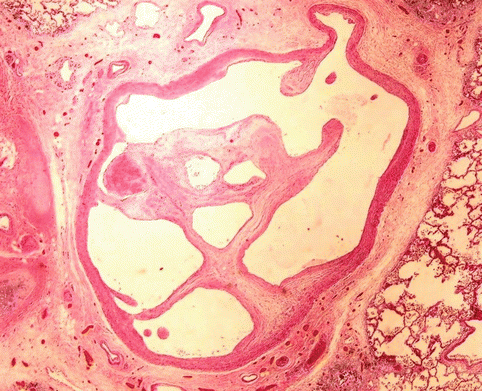

Fig. 2.10
Thrombus adhered to the vascular endothelium of the pulmonary artery

Fig. 2.11
Rechanneled thrombus with newly formed lumen
Phase I: Day 1–7 | Flowing blood on an eroded endothelium, eliciting a platelet plug and fibrin deposition with a layered growth (Zahn’s lines) |
No reaction between endothelium and thrombus is visible | |
Erythrocytes are preserved and agglomerated | |
Initial white blood cells picnosis | |
Monocytes cells with enlarged nuclei < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|