Fig. 6.1
Hospital mortality rates by sex and age among 384,878 myocardial infarction patients enrolled in the National Registry of Myocardial Infarction II. Women in the younger age groups show a higher mortality compared with men of similar age. The sex difference in mortality diminishes as patients’ age increases (Vaccarino et al. 1999)
In this chapter, we propose that psychosocial factors that are common among young and middle-aged women and that have been linked to cardiovascular risk, in particular depression, early life adversities and PTSD, may be fundamental in affecting early vulnerability to IHD in women. These factors may place women on a trajectory of high risk for IHD early in their lives, even though the disease may manifest clinically much later. This proposition should be informative in understanding current data and in developing future research centered on explaining sex differences in IHD and on developing effective interventions. For women, considerable research has focused on the years around and after menopause. We now advocate a paradigm shift towards the consideration that the pathophysiological processes leading to IHD originate premenopausally in many women. Processes that begin in the premenopausal years may be key elements, albeit neglected, of a cumulative increase in IHD risk as women age.
From Female Macaques to Women
For many years, female macaque monkeys have provided an established animal model for the study of the effects of psychological stress on coronary artery disease in women, and of the role of ovarian function in this pathway of risk. Studies of macaques and other nonhuman primates have demonstrated that chronic psychosocial stress causes endothelial damage and accelerated atherosclerosis, and, among females, ovarian dysfunction secondary to psychological stress plays a role (Kaplan and Manuck 2008). Monkeys under social stress develop endothelial injury; this effect is blocked by propranolol, the beta-adrenergic receptor antagonist, indicating that norepinephrine, a hormone involved in the stress response, is implicated. These studies have also discovered important sex differences. Among males, dominant individuals develop more extensive atherosclerosis than subordinates when housed in recurrently reorganized (unstable) social groups. By contrast, it is the subordinate females within their social group that develop accelerated atherosclerosis, rather than the dominant ones (Kaplan et al. 2009). The subordinate females are hypercortisolemic and ovary impaired, have visceral obesity and show behavioral and physiological features consistent with distress and depression (Kaplan and Manuck 2008; Kaplan et al. 2002). The accelerated atherosclerosis in subordinate young female macaques is eliminated by estrogen therapy; furthermore, ovariectomy eliminates the female protection from atherosclerosis typically exhibited by dominant animals (Kaplan and Manuck, 2008; Kaplan et al. 2002).
We anticipate that a similar model applies to premenopausal women. This question, however, is more difficult to address in humans. It is well established that stress is a major cause of ovarian compromise in premenopausal women (Ferin 1999; Chrousos et al. 1998), and stress-induced infertility, known as “functional hypothalamic anovulation” (and the accompanying hypoestrogenism), is thought to adversely affect the emotional, cardiovascular, skeletal, and cognitive health of women (Berga and Loucks 2005; Marcus et al. 2001). Over 20 % of women have some form of ovarian disruption during their reproductive years, which often goes unrecognized (Gunnell and Ewings 1994). Epidemiologic data in young women, paralleling experimental data in nonhuman primates, indicate that even mild ovarian insufficiency may result into an increased risk of coronary atherosclerosis and IHD (Kaplan and Manuck 2008).
Early Life Stress
Adverse childhood experiences, also known as early life stress or trauma, commonly include various forms of maltreatment in childhood, such as verbal, physical, and sexual abuse. Some definitions have also included family dysfunction (e.g., domestic violence, and substance abuse or incarceration of a family member), and exposure to general traumatic events (e.g., a serious accident, or the death of a parent). These events are quite prevalent in the general population. In a recent national survey of adults, approximately one quarter (26 %) reported verbal abuse in childhood, 15 % physical abuse, and 12 % sexual abuse. Sexual abuse in childhood is particularly common in women, 17 % of whom report this experience, vs. 7 % of men (Adverse Childhood Experiences Reported by Adults—Five States 2009, Centers for Disease Control and Prevention 2010). Adverse childhood experiences have been linked to a range of unfavorable health outcomes in adulthood, including substance abuse, depression, anxiety disorders, obesity, cardiovascular disease, and premature mortality (Brown et al. 2009; Danese and McEwen 2012). Recently, the association between childhood abuse and cardiovascular disease (myocardial infarction and stroke) was examined among 66,798 women enrolled in the Nurses’ Health Study 2. Severe physical abuse in childhood was reported by 9 % of the nurses and was associated with 46 % increased risk of cardiovascular disease after adjusting for other risk factors; forced sex in childhood was reported by 11 % of participants and was associated with 56 % increased cardiovascular risk (Rich-Edwards et al. 2012).
A recent meta-analysis confirmed a link between childhood maltreatment and a number of medical outcomes in adulthood, including CVD (Wegman and Stetler 2009). Despite heterogeneity of effects across studies, the association is seen both when abuse is measured by self-report and when it is measured objectively.
The relationship between early life adversities and CVD may be explained in part by behavioral and lifestyle factors, since individuals with these exposures are more likely to engage in at-risk behaviors or unhealthy lifestyle. For example, in the Nurses’ Health Study 2, the association with CVD was partially explained by behavioral factors, depression and other CVD risk factors (Rich-Edwards et al. 2012). However, evidence also links adverse childhood experiences to enduring biological changes in the nervous, endocrine, and immune systems (Danese and McEwen 2012), as described later in this chapter under “Mechanisms.”
Although few studies have examined sex differences in the relationship between early life stress and cardiovascular disease, in two populations of subjects younger than 55 years early life adversities were stronger predictors of cardiovascular events in women than in men. The United States National Comorbidity Survey is a nationally representative sample of over 5,000 adults 15–54 years of age (Korkeila et al. 2010; Goodwin and Stein 2004). Women who reported childhood maltreatment, and specifically childhood sexual abuse (Goodwin and Stein 2004), were >5 times more likely to also report heart disease in the past 12 months defined as “heart attack or serious heart trouble”, after adjusting for other demographic and behavioral factors; in contrast, no association was found among men. Furthermore, childhood maltreatment explained much of the lower rate of heart disease in women compared with men (Batten et al. 2004).
The second population of young subjects that provided data on this issue was a Finnish community sample less than 55 years of age at baseline, the Health and Social Support (HeSSup) study, a longitudinal follow-up of a random sample of the Finnish population (N = 23,916) (Korkeila et al. 2010). Among approximately 14,000 women, there were 91 confirmed cardiovascular events after about 7 years of follow-up. There was a progressive increase in cardiovascular risk with increasing number of childhood adversities among women; having three or more such exposures was associated with a threefold increased risk, after adjusting for demographic and behavioral factors, depression and other comorbidities. Again, no such relationship was seen among men (Fig. 6.2).
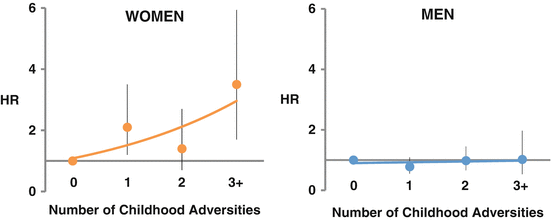

Fig. 6.2
Adjusted hazards ratios for the incidence of cardiovascular disease events according to exposure to childhood adversities among 23,916 Finish community-dwelling people age <55 years at baseline in the Health and Social Support Study. There was a progressive increase in cardiovascular risk with increasing number of childhood adversities among women but not among men (Korkeila et al. 2010)
As a whole, these data suggest that childhood social environment, in particular severe childhood adversities such as sexual abuse, is associated with adult cardiovascular risk and that these exposures are particularly significant for early-onset IHD among young women.
Depression
Depression is an important risk factor for IHD in women, increasing a woman’s risk of at least 50 % (Wassertheil-Smoller et al. 2004; Whang et al. 2009; Rosengren et al. 2004). As defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association 2000), major depressive disorder is characterized by depressed mood or loss of interest in nearly all activities for at least 2 weeks accompanied by at least four of the following symptoms: appetite or weight change, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or inappropriate guilt, diminished ability to think or concentrate, and recurrent thoughts of death. In addition, these symptoms must cause clinically significant distress or impairment in important areas of functioning. The core criteria for major depressive disorder have remained essentially unchanged in the Fifth Edition of the DSM (American Psychiatric Association 2013).
Depression is a highly prevalent condition, particularly in women. Several national population surveys have consistently shown higher (approximately from 70 % to twice as high) prevalence rates of depression in women than in men. The Substance Abuse and Mental Health Services Administration (SAMHSA) survey reported a 1-year prevalence of major depressive disorder of 8.3 % in women and 4.7 % in men (Substance Abuse and Mental Health Services Administration 2012); Alonso et al. 2004; Ayuso-Mateos et al. 2001. Women also have a more severe course of depression, with an earlier age at onset, greater severity of symptoms, and about twice as many depressive episodes as affected males (American Psychiatric Association 2000; Sullivan et al. 2000). In addition to a higher prevalence, women have approximately two times higher incidence of depression than men, with incidence rate ratios varying from 1.6 to 3.4 comparing women to men (Kuehner 2003). Interestingly, this approximate 2:1 ratio comparing women to men remains strikingly constant across countries around the world that differ largely in overall prevalence of depression (Weissman et al. 1996). Thus, some intrinsic factors may underlie the sex difference in depression, although it is likely that cultural or environmental differences affect the expression of the disorder in various settings.
Most epidemiologic studies of depression as a risk factor for IHD have examined depressive symptoms rather than a major depression diagnosis. These studies have often shown a gradient of risk linking level of depressive symptoms to likelihood of adverse cardiac events, beginning at relatively low levels of depressive symptoms. Depression also appears to be a heterogeneous condition and specific subtypes may be more strongly related with IHD, such as new-onset depression after acute coronary syndromes, treatment resistant depression, or somatic depressive symptoms as opposed to cognitive symptoms. However, there is no clear consensus on these differential effects (Zuidersma et al. 2011).
The prevalence of depression is 2–3 times higher in cardiac patients than in the general population, and among myocardial infarction patients, the prevalence of depression is distinctly high about 40 %, among younger women (<60 years old) (Mallik et al. 2006; Vaccarino et al. 2014). The higher prevalence of depression in this group suggests that young women may be more vulnerable to depression secondary to medical illness, such as an acute myocardial infarction. It may also reflect the fact that depression is a stronger risk factor for IHD in young women. Indeed, recent data suggest that the latter may be the case. In the Third National Health and Nutrition Examination Survey (NHANES III), a history of major depression and of suicide attempt (examined as a proxy for depression) was assessed via the Diagnostic Interview Schedule in 7,611 community individuals in the USA aged 17–39 years (mean age, 28 years). Mortality and cause of death information after a median follow-up of 15 years were obtained, and detailed data on traditional risk factors, socio-demographic characteristics, and health behaviors were collected. A total of 51 (0.7 %) people died from cardiovascular disease and 28 (0.4 %) from IHD. Women with either major depression or attempted suicide had more than three times the adjusted risk of cardiovascular death (a hazards ratio [HR] of 3.2) and almost 15-fold the adjusted risk of IHD (HR 14.6) (Shah et al. 2011). Corresponding figures for men were 2.4 and 3.5, with p = 0.13 for the sex interaction on IHD mortality. The attributable risk of IHD due to depression (i.e., the proportion of IHD that could be prevented if depression were to be eliminated as a risk factor from the population) in these young women was 65 %, much higher than in men (13 %), and higher than the attributable risk for traditional cardiovascular risk factors (Fig. 6.3). Although based on a small number of IHD events, these data suggest that depression is a powerful predictor of IHD in young adults, and more so in women than in men. These results are supported by other investigations. In the prospective Community Mental Health Epidemiology Study of Washington County, MD, depression increased cardiovascular risk in women younger than 40 years more than sixfold, while no association was found among men (Wyman et al. 2012). In yet another study of young and middle-aged women (mean age, 46 years), recurrent major depressive episodes were associated with a twofold risk of carotid plaques assessed by means of B-mode ultrasound (Jones et al. 2003).
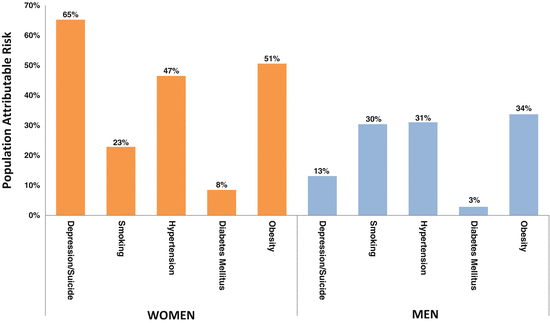

Fig. 6.3
Population attributable risk of ischemic heart disease (IHD) due to depression and traditional coronary heart disease risk factors in women and men <40 years of age at baseline in the Third National Health and Nutrition Examination Survey (n=7,611). The attributable risk due to depression (i.e., the proportion of IHD that could be prevented if depression were eliminated from the population) in women was 65 %, higher than in men (13 %), and higher than the attributable risk for traditional coronary heart disease risk factors (Shah et al. 2011)
Among women with established or suspected IHD, depression is a powerful risk factor for adverse prognosis. For example, among women referred for coronary angiography and enrolled in the Women’s Ischemia Syndrome Evaluation (WISE) study, those with elevated depressive symptoms and a previous diagnosis of depression requiring treatment had a threefold higher risk of death and cardiac events relative to women with no depression (Rutledge et al. 2006). Similarly, in a prospective study of myocardial infarction patients, an elevated level of depressive symptoms was significantly related to cardiac mortality with an odds ratio of 3.3 in women (Frasure-Smith and Lesperance 2008).
Available data indicate that men and women with IHD are similarly susceptible to the adverse effects of depression on prognosis (Frasure-Smith et al. 1999; Parashar et al. 2009). However, there are virtually no data on younger patient populations. Similar to the IHD incidence data in the NHANES III population and other cohorts described above, it is possible that depression disproportionately affects prognosis in young women with IHD. That this might be true is suggested by the fact that, among young patients with acute myocardial infarction (less than 50 or 60 years old), women differ substantially from men in their socio-demographic and psychosocial profile, including depression, while they are more similar to men in older age groups (Vaccarino et al. 1999, 2014; Mallik et al. 2006). In a recent analysis, women less than 50 years of age who had survived a myocardial infarction in the previous 6 months had a 50 % prevalence rate of lifetime history of major depression based on DSM-IV criteria; the rate among men was also high (30 %), but considerably less than in women (Vaccarino et al. 2014). The differences in psychosocial risk profile between young women and men with myocardial infarction contrasted with similarities in medical history and conventional cardiac risk factors, and, as expected, women had less severe angiographic coronary artery disease than men (Vaccarino et al. 2014). Notably, young women in this age group also show higher mortality and complication rates after an acute myocardial infarction compared with men of similar age, a difference not seen in older patients (Vaccarino et al. 1999; Rosengren et al. 2001; Andrikopoulos et al. 2006; Koek et al. 2006; Vaccarino et al. 2001). It is possible that the burden of depression and other psychosocial risk factors play a role in the adverse outcomes of young women with myocardial infarction compared with men of similar age, but this needs additional study.
Anxiety Disorders
Anxiety, like depression, is common in the general population and substantially more common in women than in men. Anxiety disorders include a large spectrum of conditions: generalized anxiety disorder, panic disorder, obsessive-compulsive disorder, phobic disorder, and PTSD. These various anxiety disorders have their own specific symptoms but also share several of them, including excessive rumination, worrying, uneasiness, tension and fear about future, real or imaginary events, and 14 % to 18 % of adult Americans may be affected by one or more anxiety disorders at some point in their lives (Kessler et al. 2005).
As for depression, most studies examining the relationship between anxiety and cardiovascular disease have considered anxiety symptom scales rather than clinical diagnoses. Results have been inconsistent and about half of the studies failed to find a significant association. A recent meta-analysis pooled the results of approximately 20 longitudinal studies and found a modest (26 %) increased risk of incident IHD and a 48 % increased risk of cardiac death for anxious persons, independent of other risk factors (Roest et al. 2010). This meta-analysis confirmed substantial heterogeneity across studies. Importantly, little is known about whether the association differs in women and men. However, based on the limited data available, general anxiety symptoms appear to be more consistent risk factors for IHD in men than in women, both for incident IHD (Ringback Weitoft and Rosen 2005; Eaker et al. 2005) and recurrent IHD events (Frasure-Smith and Lesperance 2008; Frasure-Smith et al. 1999).
Unfortunately, little information is available on specific anxiety disorders, which may differ in biological substrate and pathophysiology and thus in their relationship with IHD. Among women, recent studies have linked panic attacks (Smoller et al. 2007) and phobic anxiety (Albert et al. 2005) with incident IHD and sudden cardiac death. An additional anxiety disorder that is driving attention for a link with IHD in women (as well as men) is PTSD, as discussed below.
Posttraumatic Stress Disorder
There is emerging evidence that an anxiety disorder relatively common in women, PTSD, may increase the risk of IHD. PTSD is by definition linked to a traumatic event, which in turn is defined as a threat to one’s life or self-integrity. PTSD is characterized by recurrent memories of the traumatic event (intrusions) that the patient cannot control, such as nightmares and feeling worse with reminders of the event; avoidance symptoms, namely avoiding things that would remind the person of the trauma, and having difficulty remembering specific past experiences; and hyper arousal, including trouble falling or staying asleep, irritability, outbursts of anger, and difficulty concentrating. An increased startle response, for instant being jumpy with loud noises, is another symptom of hyper vigilance. More than half of the population will experience a traumatic event at some point in their lives and is therefore at risk of PTSD (Reed et al. 2012). For women the most common type of trauma is sexual abuse or assault, while for men it is physical assault (Reese et al. 2012). PTSD is twice as common in women as it is in men. The lifetime prevalence tends to be higher in the United States (10–12 % in women and 5–6 % in men) than in Western Europe (< 3 % in women and < 1 % in men) (Alonso et al. 2004), although PTSD rates in some European countries, such as the Netherlands and Denmark, are similar to the United States (de Vries and Olff 2009; Elklit 2002). Rates are much higher around the world in post-conflict settings (de Jong et al. 2001).
The prospective association between PTSD and IHD has been examined in only five studies. Four of these investigations included predominantly male veterans and found a significant association between PTSD symptoms or a PTSD diagnosis with fatal or nonfatal IHD events (Kubzansky et al. 2007; Boscarino 2008; Ahmadi et al. 2011; Vaccarino et al. 2013a), as well as objective indicators of coronary heart disease such as coronary artery calcium, myocardial perfusion defects (Ahmadi et al. 2011; Vaccarino et al. 2013a) or other indicators of myocardial ischemia (Turner et al. 2013). Only one prospective study has examined women. Among 1,059 women free of IHD at baseline, those with five or more symptoms of PTSD had over threefold risk of IHD compared with those without PTSD symptoms after a follow-up of around 14 years (Kubzansky et al. 2009). Results were maintained after adjusting for standard coronary risk factors and also depression and trait anxiety.
Potential mechanisms
Several potential mechanisms have been postulated for the relationship between psychosocial risk factors and IHD. Many of these mechanisms are shared by various psychosocial risk factors, although some differences exist. Unfortunately, despite a relatively large mechanistic literature linking psychosocial stress to IHD as well as cardiometabolic risk factors, limited data exist specific for women.
Behavioral factors. People with psychosocial risk factors such as depression, childhood trauma, and PTSD are more likely to follow unhealthy lifestyle and to engage in adverse behaviors such as smoking, sedentary lifestyle, delay in seeking treatment and nonadherence to secondary prevention measures (Whooley et al. 2008; Carney et al. 1995; Breslau et al. 2003). In coronary heart disease patients, psychosocial burden is also associated with severity of functional impairment (Ruo et al. 2003), which may exacerbate physical inactivity and poor self-care. The association between psychosocial risk factors and IHD, however, usually persists after controlling for health behaviors, indicating that the latter only partially explain the association.
Neuroendocrine abnormalities. All the factors discussed in this chapter have been associated with neuroendocrine and autonomic dysregulation, which may result in an adverse cardiometabolic risk profile and increased IHD risk. Neuroendocrine dysfunction and disturbances in autonomic cardiac control are likely to be implicated as mechanisms (McEwen 2008).
Preclinical and clinical studies demonstrate profound and lasting adaptive changes secondary to early stress exposure on neurobiology, including abnormal glucocorticoid and norepinephrine function, heightened autonomic stress reactivity, and altered dopaminergic and serotonergic function (Danese and McEwen 2012; Bremner 2003). This enduring vulnerability to biological disruption is referred to as “biological embedding” (Danese et al. 2011). In laboratory studies, early life stress leads to heightened stress reactivity and alterations in neural circuits involved in the stress response [the hypothalamic-pituitary-adrenal (HPA) axis and the noradrenergic system] that persist into adulthood. Adult rats that were separated from their dams for 180 min/day on postnatal days 2–14 exhibit up to threefold increases in adrenocorticotropic hormone (ACTH) and corticosterone responses to psychological stressors compared to control rats (Ladd et al. 2000; Plotsky and Meaney 1993). Early life stressors also lead to long-term abnormalities in the noradrenergic/locus coeruleus system with increased noradrenergic responses to stressors (Sanchez et al. 2001; Caldji et al. 1998). Similarly, nonhuman primates exposed to prolonged periods of maternal or social deprivation exhibit marked behavioral, physiological, and neurobiological changes that are maintained in adult age (Gilmer and McKinney 2003).
In humans, early life stress plays a major role in increasing the risk of depression and PTSD and is associated with long lasting neurobiological perturbations particularly among women. Women abused as children exhibit greater plasma ACTH, cortisol, and heart rate responses to a psychosocial laboratory stressor than controls (Heim et al. 2000). Not only are women more susceptible towards experiencing childhood abuse, but also towards depression as a consequence of it, because the female HPA axis appears to be distinctly vulnerable to dysregulation induced by psychological stress. A history of childhood maltreatment has also been associated with smaller volumes of the prefrontal cortex and the hippocampus, greater activation of the HPA axis during stress, and elevated inflammation (Bremner 2012). These changes persist in adulthood, many years after these early exposures, providing evidence for an enduring effect of early life stress on physical health (Danese et al. 2011).
Depression, which, as described above, in women is often secondary to early life stress, is also characterized by hyperactivity of the neuroendocrine stress systems, including the HPA axis and the sympathoadrenal system, resulting in increased, or prolonged, release of cortisol and norepinephrine and disruption of normal circadian patterns. Depressed individuals also show reduced parasympathetic flow, which contributes to autonomic nervous system dysregulation (Carney et al. 2005a).
PTSD, on the other hand, is characterized by chronic dysregulation of the HPA axis with increased corticotrophin releasing factor levels and decreased peripheral cortisol concentrations at rest (Bremner and Charney 2010). PTSD is also associated with increased activation of the sympathetic nervous system during reminders of the trauma, as shown by higher catecholamine levels after exposure to traumatic reminders compared with controls (Blanchard et al. 1991; McFall et al. 1990; Yehuda 2002). Elevated catecholamine response to trauma-reminiscent cues may lead to hemodynamic hyperactivity during everyday life, which may eventually affect cardiovascular health (Pitman et al. 1990; Kleim et al 2010). Indeed, combat veterans with PTSD, compared with controls, exhibit an increase in heart rate and other physiological parameters in response to auditory reminders of trauma (such as tapes of the sound of gunfire), combat slides, or scripts of the individual’s traumatic experiences (Pitman et al. 1990; Blanchard et al. 1982). In contrast, no increase in heart rate in response to neutral stressors such as mental arithmetic is found in PTSD. Repeated sympathetic system responses to trauma reminders could affect myocardial electrical stability and the risk for cardiac arrhythmias (Lane et al. 2005; Lampert et al. 2002), and could contribute to reduced heart rate variability and baroreflex function, important risk factors for cardiac events (Bigger et al. 1992; La Rovere et al. 1998). Heart rate variability is indeed decreased in PTSD, independent of depression and other risk factors (Shah et al. 2013).
Alterations in neurohormonal stress response systems contribute to disruption of key neuroendocrine and immune function pathways involved in physiological homeostasis. A commonly endorsed risk pathway is “wear and tear” of the cardiovascular system due to sustained or repeated sympathetic nervous system stimulation and parasympathetic nervous system withdrawal. These abnormalities may lead to repeated or sustained elevations in blood pressure, heart rate, plasma glucose, insulin resistance, and dyslipidemia. However, it seems unlikely that these risk factors entirely account for the relationship between depression and CVD risk, because when adjusted for statistically, the relationship remains (Nicholson et al. 2006). Increased inflammation, endothelial dysfunction, and hypercoagulability may also play a role, as described below (McEwen 2008).
Inflammation, coagulation, and endothelial function. Dysregulation of neurohormonal stress response systems may contribute to immune dysfunction and inflammation, which are implicated both in the physiological response to stress and in the pathophysiology of IHD. Inflammatory processes are linked to a hypercoagulable state and to endothelial dysfunction, which are also mechanisms for IHD. A direct link between psychosocial stress and inflammation has been described in experimental studies showing that stress-induced norepinephrine activates the transcription factor NF-kB in circulating monocytes, initiating the inflammatory cascade (Bierhaus et al. 2003).
Such effects can be quite pronounced and enduring, resulting in long lasting abnormalities in immune functioning. In a prospective study, maltreated children showed higher inflammation 20 years later, which persisted after accounting for other childhood exposures and health behaviors (Danese et al. 2007).
Depression and PTSD have been associated with higher levels of inflammatory biomarkers and evidence of immune dysregulation (von Kanel et al. 2007; Spitzer et al. 2010; Hoge et al. 2009; Uddin et al. 2010; Empana et al. 2005; Vaccarino et al. 2007; Lesperance et al. 2004). Enhanced platelet activity in depression has long been proposed as a potential link between depression and cardiac events, but data are limited and results mixed (von Kanel 2004). Endothelial dysfunction in depression has also been described (Sherwood et al. 2005), and limited evidence also relates PTSD symptom severity to endothelium-derived circulating proteins and blood clotting factors (von Kanel et al. 2006, 2008).
Genetic and epigenetic factors. Growing evidence suggests that some psychosocial factors, particularly depression, may share genetic pathways with IHD (Mulle and Vaccarino 2013). This means that depression and IHD may be, at least in part, different phenotypic expressions of the same genetic substrate (de Geus 2006; Kendler et al. 2009; Vaccarino et al. 2009). Such a paradigm may be particularly true for women, as initially pointed out by analyses of the Swedish twin registry, including 15,284 twin pairs of which 53 % were female (Kendler et al. 2009). This study assessed the temporal relationship of the depression-cardiovascular disease comorbidity, and found that it was reciprocal, and modified strongly by gender. Onset of cardiovascular disease predicted future depression risk, and onset of depression increased future cardiovascular disease risk, supporting a model of common factors contributing to the risk for both disorders. However, for males shared genetic factors contributed to the depression-cardiovascular disease comorbidity in younger age groups only; in older men, this comorbidity was mediated largely by environmental effects. In contrast, among women common genetic factors contributed to this comorbidity across all age groups (Kendler et al. 2009). This study established that genetic susceptibility to a psychosocial factor can be modified by gender. Notably, genetic influences on depression are estimated to be stronger in females compared to males; the heritability of depression has been estimated at 42 % in women but only 29 % in men (Kendler et al. 2001, 2006). Thus, as a whole, the above data suggest that the link between depression and IHD in women is more often attributable to genetic factors than in men.
Genes related to inflammation, platelet aggregation, and the serotonin system may be especially relevant to the comorbidity of depression and cardiovascular disease. An area where sex differences may take place involves inflammatory genes. Leukotrienes are the most potent inflammatory mediators and their regulation is essential for normal brain function. A recent study found that a haplotype composed of six single nucleotide polymorphisms (SNPs) in the leukotriene A4 hydrolase gene showed a significant protective effect towards depression in women, but not in men (Zhao et al. 2009). This same haplotype was protective towards coronary artery disease severity in women only, and 7 % of the association between depression and coronary artery disease in women was explained by this haplotype.
Evolving knowledge also links psychosocial stress, psychiatric disorders, and cardiovascular disease through epigenetic mechanisms (Uddin et al. 2010; Baccarelli et al. 2010; Mehta et al. 2013). Epigenetic changes include modifications of the superstructure of the chromatin through molecular mechanisms such as DNA methylation and histone modifications, which result in modulation of gene expression without altering the DNA sequence (Egger et al. 2004). Aberrations in DNA methylation have been implicated in the etiology of various mental disorders, including depression (Fuchikami et al. 2010) and PTSD (Uddin et al. 2010), but also play a role in the pathophysiology of physical illness including cardiovascular disease (Baccarelli et al. 2010). Thus DNA methylation provides a potential biological basis for gene–environment interactions relevant to both mental health and chronic diseases, for example, interactions between genes and stress exposure. Epigenetics also offers a biological substrate for modifications in gene expression that may affect multiple phenotypes—in our case, both mental health and cardiovascular disease. The evidence for epigenetic changes is especially robust for early life stress. Many animal and human studies have found that early life experiences can alter DNA methylation and affect gene expression and behavior, and influence susceptibility to future disease (McGowan et al. 2009; Meaney and Szyf 2005). Similarly, experiences later in life can modify the epigenome (Wong et al. 2010; Fraga et al. 2005). A recent study also reported changes in DNA methylation in the oxytocin receptor (OXTR) gene immediately after acute psychosocial stressor challenge (Unternaehrer et al. 2012).
Acute response to stress and mental stress ischemia. Psychosocial factors could increase IHD risk by inducing silent ischemia, triggering acute coronary syndromes, or heightening cardiovascular reactivity in daily life. For example, depressive mood has been shown to trigger ischemia detected by ECG ambulatory monitoring in patients with coronary disease (Gullette et al. 1997). This effect, possibly mediated by the autonomic nervous system, may be secondary to increased oxygen demands on the heart or stress-related coronary vasoconstriction (Rozanski et al. 1999).
Emerging data suggest that myocardial ischemia due to emotional factors, commonly referred to as “mental stress ischemia,” but not exercise-induced ischemia, is more common in women survivors of an acute myocardial infarction up to 50 years of age compared with age-matched men (Vaccarino et al. 2014). In this study, myocardial ischemia was assessed with myocardial perfusion imaging, and results were not explained by differences in traditional coronary risk factors, severity of coronary artery disease, or behavioral and psychosocial factors. Most other studies of mental stress ischemia were performed predominantly in men, and when women were included, the female sample was often too small to be examined separately. In addition, the study populations included a broad set of coronary disease diagnoses rather than patients with a previous myocardial infarction, a subgroup where women face higher risks. Another recent study where mental stress-induced ischemia was assessed by echocardiography found almost two times higher odds of mental stress ischemia in women than in men, although the difference was not significant in multivariable analyses (Jiang et al. 2013). This study, again, included a broad range of patients with stable coronary artery disease, the majority of whom were older men with no prior history of myocardial infarction.
It is unclear why myocardial ischemia with mental stress is more common in women after a myocardial infarction, specifically in younger age groups. This finding is not due to more pronounced hemodynamic responses to stress in female patients, as hemodynamic data are similar in women and men (Becker et al. 1996; Steptoe et al. 1996; Vaccarino et al. 2014). A possible mechanism is a higher burden of psychosocial risk factors in women. Indeed, younger women with myocardial infarction show a more adverse psychosocial risk profile. They are more often poor, of minority race, more depressed, and with a frequent history of trauma compared with men and with older women (Vaccarino et al. 1999, 2014; Mallik et al. 2006).
Vasomotor tone could also be an important substrate for women’s susceptibility to mental stress ischemia. Women appear to be more vulnerable to abnormal vasomotor reactivity and micro-vascular dysfunction in the coronary bed than men, to the point that these have been proposed as female-specific mechanisms of IHD and prognostic factors (Wong et al. 2002; von Mering et al. 2004; Pepine et al. 2010). In turn, there is a wealth of data pointing to endothelial and micro-vascular dysfunction as correlates of mental stress ischemia, possibly through sympathetic nervous system activation via adrenergic receptors in the vascular system. For example, mental stress has been associated with paradoxical vasoconstriction or reduced blood flow in coronary vessels (Arrighi et al. 2000; Kop et al. 2001). Peripherally, even a brief period of mental stress induces prolonged endothelial dysfunction in the brachial artery (Ghiadoni et al. 2000). Furthermore, patients with IHD who develop mental stress-induced ischemia consistently show an increase in systemic vascular resistance (Goldberg et al. 1996; Jain et al. 1998; Burg et al. 2009), suggesting that a rise in afterload secondary to peripheral vasoconstriction plays a role in mental stress ischemia. Thus, the fact that vascular dysfunction is an important mechanism of mental stress ischemia could explain a female predominance of this phenomenon in post-myocardial infarction patients.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


