Fig. 9.1
Cardiovascular disease mortality in the Netherlands in 2011, stratified by age cohorts and sex (CBS 2013a)
Evidence indicates considerable differences between women versus men in the prevalence of coronary artery disease (CAD) as related to its clinical manifestations. Approximately half of the women with chest pain do not have significant CAD as measured by coronary angiography (i.e., <50 % luminal narrowing of a coronary artery), which is much more than the 17 % absence of significant CAD in men (Shaw et al. 2002). Similar findings have been reported by Jespersen et al. with 48 % of women versus 19 % of men having “normal” coronary arteries at angiography, whereas no sex differences were found in diffuse non-obstructive lesions (17 % versus 14 %, respectively) (Jespersen et al. 2012). A similar pattern was reported by Sedlak and colleagues: 30 % non-obstructive CAD in women vs. 10 % in men (Sedlak et al. 2013). Because of the later onset of symptoms by approximately 10 years and the lower rate of obstructive CAD in women the incorrect notion that CHD is a “man’s disease” remains persistent.
Symptoms of chest pain and pressure are associated with CHD in men with relatively good sensitivity and specificity, but in women these symptoms are not as sensitive or specific (Bairey Merz et al. 2006; McSweeney et al. 2003). When diagnosis is based on “typical” symptoms of chest pain, approximately two-thirds (65 %) of women who actually have CHD are not identified as having CHD (Bairey Merz et al. 2006). Instead, the most common acute symptoms of CHD in women are shortness of breath, weakness, and fatigue (McSweeney et al. 2003). One of the consequences of the different symptom profiles in women is that the patient as well as physicians and other healthcare providers have to differentiate their interpretations of these symptoms from other conditions such as inflammation-related diseases and psychological distress and anxiety. These “competing interpretations” may result in delayed and often missed diagnosis of CHD and lack of initiation of necessary clinical interventions (Bairey Merz et al. 2006; Chiaramonte and Friend 2006; Roger et al. 2000; Vaccarino et al. 2013).
The term “ischemic heart disease” (IHD) is preferable over CHD when describing heart disease in women (Vaccarino et al. 2013). With IHD the importance of myocardial ischemia is implied without requiring involvement of significant obstructive narrowing of the epicardial coronary arteries (i.e., the larger conduit arteries on the heart muscle that supply the heart mucle with oxygenated blood). This is consistent with the relatively lower prevalence of CAD in women combined with the comparable levels of myocardial ischemia (Vaccarino et al. 2013). The clinical phenotype of IHD in women can present as angina (exercise induced chest pain) related to CAD, or as other symptoms such dyspnea, fatigue, nausea, or sweating that reflect myocardial ischemia resulting from microvascular disease, CAD, or a combination of these conditions. Microvascular ischemia is characterized by abnormal vascular function in “resistance vessels” of the heart, i.e., the microvasculature. This condition can coexist with endothelial dysfunction in the epicardial coronary arteries. Endothelial dysfunction involves poor function of the lining cells of the coronary vessels (i.e., the endothelium) and is common at all stages of CAD. Endothelial dysfunction can result in transient vasoconstriction and subsequent myocardial ischemia and can be present in vessels without luminal narrowing. Endothelial dysfunction may therefore explain why significant obstructive CAD (i.e., luminal narrowing >50 %) is often not detected in women with cardiac symptoms and why non-obstructive CAD is not necessarily benign.
In this context it is relevant to consider cardiac syndrome X. Cardiac syndrome X is characterized by: (1) inducibility of myocardial ischemia as documented by ECG changes, transient wall motion abnormalities, or perfusion defects on cardiac imaging following exercise or pharmacological provocation tests; (2) a history of chest pain or other cardiac symptoms; and (3) normal or minimally narrowed coronary arteries (<50 % luminal stenosis) at angiography (Arthur et al. 2012; Kemp et al. 1973). It is possible that cardiac syndrome X involves both epicardial coronary dysfunction as well as microvasculature dysfunction. Both pathophysiological processes may lead to reduced blood flow and cause myocardial ischemia and symptoms that are qualitatively different from obstructive CAD (Bairey Merz et al. 2006; Panting et al. 2002). These conditions require further investigation as the differential diagnosis is complicated and treatment options are not well developed. In addition, the standard cardiovascular risk evaluations (e.g., the Framingham Risk Factor Score) (D’Agostino et al. 2008) are suboptimal for risk stratification in women and alternative risk scores have been proposed that include inflammation markers such as high sensitivity C-reactive protein (hs-CRP) (Ridker et al. 2007). The role of inflammation and other immune system-related processes may play a critical role in the observed sex differences in IHD and its clinical manifestations. These immune system-related processes are influenced by psychological, neurological, and hormonal factors. This chapter selectively reviews the role of psychosocial distress and sex differences in IHD, inflammation-related processes in atherosclerosis, psychoneuroimmunological (PNI) processes in IHD, and the role of estrogens in IHD and PNI-related mechanisms of atherosclerosis. These topics are presented in relation to the differences in heart disease in women versus men.
Psychosocial Distress and Sex Differences in IHD
Multiple studies have demonstrated that psychological and social factors are associated with increased risk of IHD (Dimsdale 2008; Suls and Bunde 2005). Both depression and anxiety are associated with an increased risk of mortality in individuals with CAD (Frasure-Smith and Lesperance 2008; Nicholson et al. 2006; Roest et al. 2010; van Melle et al. 2004). In addition to depression and anxiety, the main psychosocial factors associated with an elevated risk of IHD are anger/hostility, (low) social support, (low) socioeconomic status, and general psychological distress related to family, work, care giving, among others. Because the association between depression and cardiovascular disease is well established, and the knowledge based on changes in presence of depression throughout the life cycle in women, this chapter primarily focuses on depression and its biological correlates relevant to IHD in women. Sex differences related to depression-associated risk of IHD remain under-investigated (Moller-Leimkuhler 2008).
Depression is the leading cause of global burden of disease in high income, and third cause of global burden in low- and middle-income countries (Ferrari et al. 2013; WHO 2008). Women are more likely to present with psychological complaints compared to men, including depression, anxiety, and general distress (Faravelli et al. 2013; Ferrari et al. 2013a, b; WHO 2008). Over the lifecycle, differences in prevalence of psychological distress between men and women emerge in mid-puberty, show a steady higher prevalence in women up until perimenopause and differences decline in older adulthood, after menopause (Faravelli et al. 2013; Ferrari et al. 2013a).
The change in depression over the lifecycle is displayed in Fig. 9.2, showing the number of women and men receiving treatment for depression in the Netherlands in 2011, divided into age categories (Fig. 9.2) (CBS 2013b). This figure shows the increased prevalence of depression in women compared to men over the lifecycle, as well as the decline in older adulthood for women as well as men. The prevalence across the lifecycle may suggest an effect of sex hormones on mood. Indeed, during reproductive years an increase in the frequency of depressive symptoms is observed, purportedly related to cyclic changes in sex-steroid hormones (e.g., related to the menstrual cycle, infertility treatment, or giving birth: post-partum depression), and the perimenopausal phase in which estrogen levels fluctuate substantially (Douma et al. 2005; Soares 2013; Williams et al. 2007). This suggests a “window of vulnerability” for depression during the female reproductive lifecycle, in which fluctuating levels of estrogens may play a role. In line with this hypothesis, lower levels of depressive symptoms have been found in women using oral contraceptives between 25 and 34 years, which may be explained by the stabilizing effects on fluctuating hormone levels (Keyes et al. 2013).
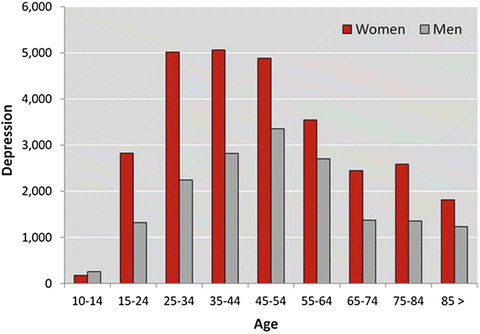

Fig. 9.2
Mental health treatment for depression in the Netherlands in 2011, stratified by age and sex (CBS 2013b)
Menopause is defined as the absence of menstruation for 12 consecutive months. Perimenopause, or menopausal transition, is the period preceding menopause, that in most women has a duration of 3–4 years but can last up to 10 years. Perimenopause generally starts between 40 and 55 years of age (Gibbs et al. 2012). During the menopausal transition, pituitary signaling of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) to the ovaries and the ovaries signaling estrogen and progesterone back to the brain becomes irregular, resulting in high fluctuating levels of estrogen. The peri- and postmenopausal phase can be accompanied by climacteric symptoms, including irregular periods with reduced or increased bleeding, hot flashes or night sweating (also known as vasomotor symptoms), sleep disturbances, mood symptoms, concentration difficulties, and/or vaginal dryness as a consequence of a reduction in estrogen levels (IQWiG 2013). Not all symptoms are present, and there are large inter-individual differences in experience and duration of these symptoms.
Depressive symptoms are reported to increase in the peri-and postmenopausal phases compared to pre-menopause (Bromberger et al. 2010; Freeman et al. 2004; Weber et al. 2014). This increase is observed in women with no history of depression as well as those with a past history of major depression disorder (Worsley et al. 2012). However, findings are not consistent as both increased, as well as decreased depressive symptoms have been observed in the menopausal transition phase (Llaneza et al. 2012). There is a more clear association between presence of climacteric symptoms, especially vasomotor symptoms, with depressive symptoms (Llaneza et al. 2012), but conflicting evidence is reported as well (Worsley et al. 2012). Furthermore, there are shared etiological factors for depression as well as age of menopause, such as low education level, financial strain, and poor self-rated health (Gold et al. 2013). Evidence for a direct association between depressive symptoms and hormonal levels, e.g., estrogen, or androgens as DHEA(S) is inconsistent (Llaneza et al. 2012; Worsley et al. 2012), which suggests that other pathways are likely to mediate the association between peri- and menopausal changes and depressive symptoms.
The increased prevalence of depression in women is also observed in patients with IHD, with a higher frequency in women (19 %) compared to men (12 %) (Shanmugasegaram et al. 2012). Some evidence suggests that depression is only associated with future CHD-related mortality in men but not in women, whereas depression predicts IHD incidence in both men and women (Ferketich et al. 2000). However, these sex differences are not consistently found across studies. Findings from the Women’s Ischemia Syndrome Evaluation study showed that depression predicted hospitalization and mortality in women with suspected IHD (Rutledge et al. 2006). Similar findings were observed in other large epidemiological cohorts in women, such as The Stockholm Female Coronary Angiography Study, showing that women with high stress related to work or family challenges develop narrowing of the coronary arteries (Wang et al. 2007). Low and colleagues reviewed 67 reports on sex differences in psychosocial risk factors for CHD incidence or recurrence. Results indicated that depression, anxiety disorders, and stress related to family issues are associated with an elevated CHD risk among women, whereas the associations of general anxiety, work-related stress, and hostility are less clearly associated with the risk of CHD in women compared to men (Low et al. 2010).
In summary, psychological risk factors are elevated among women, particularly depression, in the reproductive phase of the lifecycle. This trajectory of depression across the life cycle does not correspond with the relatively low risk of CHD in women during this time period (Figs. 9.1 and 9.2). Depression is also a less consistent predictor of CHD-related mortality in women compared to men, which may in part reflect these different trajectories across the lifespan. It is possible that the associations between psychological risk factors for IHD are differentially associated with biological processes in men versus women, which may have consequences for IHD pathophysiology. Inflammation and other immune system-related processes play a key role in early stages of CAD and also in the transition from stable CAD to clinical syndromes such as myocardial infarction. In the next section we will summarize the role of the immune system in CAD and provide a PNI perspective on CAD, which has implications for the onset and progression of IHD in women.
Inflammation-Related Processes in Atherosclerosis
It is important to distinguish between gradual CAD progression and its clinical manifestation such as myocardial infarction and CHD-related death because the immune system influences these conditions via different pathways. Atherosclerosis is a gradual disease process developing from minor injury to the endothelium and fatty streaks, to damage to the intima (atheromata). Subsequently, smooth muscle cells migrate into the early lesion and proliferate to result in a complex atherosclerotic plaque. Inflammation-related processes play a critical role at all stages of the atherosclerotic disease process (Danesh et al. 1997; Fahdi et al. 2003; Hansson 2005; Libby and Theroux 2005). Because atherosclerosis results in damage to the coronary vessel wall, the gradual progression of atherosclerotic plaques can be viewed as a “response to injury” (Ross 1986).
At the early stages of gradual CAD progression, the immune system plays a role in the adhesion of circulating monocytes, lipids, and T-cells to the vascular wall and the initial development and progression of the atherosclerotic plaque. These circulating monocytes adhere to and infiltrate the lining of coronary arteries via upregulation of intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Increased expression of ICAM and VCAM are also associated with increased leukocyte interaction and migration of leukocytes from the bloodstream to the intracellular matrix, contributing to local inflammatory processes. As coronary atherosclerotic lesions progress, they are covered with a fibrous cap which tends to become increasingly stable over time. The engulfment of lipids, monocytes, and T-cells produces damage to the vascular wall and results in an immune system-mediated response to this injury. The differentiation of monocytes into macrophages in the vascular wall is an essential aspect of atherosclerosis. These macrophages activate pattern-recognizing receptors for innate immunity (i.e., upregulation of scavenger receptors and toll-like receptors). Macrophages affect atherosclerosis in several ways by: (1) increasing the uptake and metabolism of lipids; (2) oxidation of low density lipoprotein (oxLDL) cholesterol; (3) enhancing smooth muscle cell proliferation; and (4) promoting the production of toxic atherogenic substances (products of lipid oxidation; free radicals). Oxidized lipids purportedly interfere with the normal phagocytic and scavenger function of macrophages (Adams 1994; Libby and Hansson 1991). This may lead to macrophageous foam cells in the vascular wall, lipid-laden smooth muscle cells, and extracellular lipid deposits. Activated macrophages also release cytokines and proteases (protein-breakdown enzymes), potentially leading to inflammation and tissue damage (Hansson 2005).
In addition to T-cells, B-cells may contribute to the atherosclerotic disease process. B-cells are not commonly prevalent in atherosclerotic lesions and may have anti-atherosclerotic properties. The production of auto-antibodies against oxLDL by activated B-cells is part of the adaptive immune response, which makes the atherosclerotic process in part an autoimmune condition (Fairweather et al. 2012). The prevalence of cardiovascular disease is indeed higher in people with autoimmune disorders (Kresanov et al. 2013).
In addition to these processes relevant to gradual CAD progression, activation of the immune system adversely affects plaque stability by different processes (e.g., via metalloproteases and factors that destabilize the fibrous cap). Plaque activation and rupture are critical features of myocardial infarction and CHD-related mortality. The imbalance between blood coagulation and fibrinolytic processes may result in thrombus formation and may interact with plaque instability to result in acute coronary syndromes (Arbab-Zadeh et al. 2012). The disruption of relatively small atherosclerotic plaques can play a crucial role in the pathogenesis of acute coronary events as these plaques are relatively unstable and collateral supply vessels may not have developed.
The observations described above are largely based on histopathological studies. The role of inflammation is supported by clinical-observational and epidemiological investigations. Epidemiological studies have shown that markers of inflammation are associated with an increased risk for cardiovascular events (Kuller et al. 1996; Liuzzo et al. 1994; Ridker et al. 1997). The predictive value of CRP, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) are well established (Danesh et al. 1997). CRP is an acute phase protein synthesized in the liver in response to inflammation. CRP levels are increased in inflammatory conditions such as an acute infection, surgery, or in chronic autoimmune diseases (Lavie et al. 2009) and CRP also plays a role in atherogenesis as well as plaque instability. A meta-analysis of 22 studies found a significant risk associated with CRP for incident and recurrent myocardial infarction and CHD-related death (OR = 1.5) (Cossette et al. 2013; Danesh et al. 2004; Lavie et al. 2009), although bias and heterogeneity in the reported associations have been documented (Hemingway et al. 2010). Nonetheless, elevated hs-CRP (levels ≥ 3 mg/L) adds to a cardiovascular risk profile, and is now incorporated in the Reynolds Risk score. The Reynolds Risk score was developed in order to improve the global cardiovascular risk in women (Ridker et al. 2007). In patients with intermediate risk for CAD who have elevated CRP levels, cholesterol lowering statins reduce CRP levels, and subsequent cardiovascular risk (Lavie et al. 2009).
Thus, the immune system is involved in both the gradual development of CAD as well as plaque instability and subsequent acute coronary events. At early stages of atherosclerosis, the immune system acts via effector molecules that accelerate lesion progression, whereas plaque activation and rupture play a role in myocardial infarction and CHD-related mortality. There are also indirect pathways by which the immune system affects CAD progression, as most CAD risk factors have inflammatory correlates (e.g., hypertension, diabetes, smoking, dyslipidemia, and obesity). The immune system-related measures described above are also associated with psychological risk factors for IHD. This background therefore suggests that the paradigm of psychoneuroimmunology is potentially relevant to IHD in general and sex differences in IHD in particular.
Psychoneuroimmunological Processes in Ischemic Heart Disease
The inflammatory response is part of the integrated general “stress response” to environmental challenges (Black 2003). The associations between psychological factors associated with increased risk of IHD and the immune system are complex and involve bi-directional pathways. Prolonged psychological distress, exhaustion, and depression are associated with immunosuppression (Kop and Gottdiener 2005; Segerstrom and Miller 2004). The immune system correlates of prolonged psychological distress include increased levels of leukocytes, cytokines (e.g., IL-6 and TNF-alpha), systemic inflammation markers (e.g., CRP), and antibodies to viruses (e.g., cytomegalovirus (CMV)). These factors are largely pro-inflammatory and are also involved in CAD progression and its clinical manifestations such as myocardial infarction. It could be hypothesized that atherosclerosis may initially be promoted by immunosuppressive effects of persistent psychological distress, whereas at later stages of atherosclerosis immune system activation may increase plaque instability and subsequent plaque rupture (Fricchione et al. 1996; Kop and Cohen 2007).
The central nervous system modulates immune system function primarily via the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system. Psychological distress and depression are in most instances associated with elevated corticotrophin releasing hormone (CRH) levels, resulting in a wide range of biological response including the release of pro-inflammatory cytokines. The parasympathetic outflow tract of the autonomic nervous system inhibits macrophage activation and release of pro-inflammatory cytokines via the “cholinergic anti-inflammatory pathway” (Huston and Tracey 2011; Tracey 2002). Reduced parasympathetic nervous system activity observed in CAD patients with depression may contribute to the elevated levels of pro-inflammatory cytokines (Kop et al. 2010). The association between psychological distress and depression with immune system parameters has been reviewed in detail elsewhere (Howren et al. 2009; Kop and Gottdiener 2005; Segerstrom and Miller 2004).
Our group and others have consistently found elevated CRP levels among individuals with depressive symptoms (e.g., Empana et al. 2005; Kop et al. 2002). Patients with depression and/or exhaustion also have increased levels of antibody to several herpes viruses, including CMV (Appels et al. 2000; Miller et al. 2005). The associations between psychological distress and inflammation markers are mediated, in part, by being overweight and other adverse health behaviors. Approximately 10–15 % of the association between depression and CHD-related mortality can be explained by inflammation-related processes (Kop et al. 2010; Whooley et al. 2008).
As mentioned in the introductory paragraphs, the clinical presentation of IHD in women is in most instances not “typical” chest pain, but rather involves fatigue, feelings of general malaise, and dyspnea. It is possible that IHD-related inflammatory processes contribute to the central nervous system function and states similar to depression and exhaustion (Dantzer and Kelley 1989; Goodkin and Appels 1997). For example, administration of cytokines (e.g., IL-1 and TNF-alpha) can indeed activate the HPA-axis (Sapolsky et al. 1987) and this negative feedback loop may contribute to the immunosuppressive state in depression. Thus, the symptoms of fatigue and malaise in IHD may partially reflect an adaptive response by conserving energy resources in the setting of elevated distress and/or underlying IHD-related biological processes. It is not known to what extent the more frequent complaints of fatigue and malaise prior to myocardial infarction in women than men is attributable to chronic low-grade inflammation and other immune system-related processes that are more prevalent in women than men (Cutolo and Calvia 2007).
Information about psychological traits as related to the PNI components of CAD is less extensive compared to depression. It is possible that these “traits” promote early CAD progression by adversely affecting the normal phagocytic function of macrophages (Adams 1994), which would be consistent with the observation that hostility is associated with a higher percentage of monocytes among patients with CHD (Gidron et al. 2003). Research has also shown that Type D personality, a combination of high trait negative affectivity and social inhibition, is associated with higher TNF-α and soluble TNF-α receptor-1 levels in patients with heart failure (Denollet et al. 2003; Mommersteeg et al. 2011). In addition to trait anger, hostility, and Type D personality, low socioeconomic status may act as an additional source of sustained exposure to psychological distress. Low socioeconomic status is associated with adverse cardiovascular health outcomes, which is mediated in part by increased cardiovascular risk factors and adverse health behaviors (Kraus et al. 1980; Winkleby et al. 1992), including measures of chronic low-grade inflammation (Owen et al. 2003). These chronic psychosocial factors, particularly trait anger and hostility, may also be important factors in the triggering potential of environmental stressors for myocardial infarction and CHD-related death. For example, hostility is associated with elevated levels of innate natural killer (NK) cells during mental challenge tasks (Mills et al. 1996), although results in that study are difficult to interpret as the overall response was in the direction of immune suppression and findings were not entirely consistent across the responses of the immune system measures.
In summary, immunosuppression of the adaptive immune system is well documented in psychological risk factors for IHD, in particular depression. The relationship between these psychological factors and immune system parameters is complex, involves multiple feedback mechanisms and is bi-directional. Evidence indicates that the central nervous system correlates of psychological distress and depressive symptoms can lead to immune system changes, and a reverse pathway has been documented as well. The pro-inflammatory state that is often observed during episodes of elevated psychological distress and depression may promote gradual CAD progression by macrophage and lipid engulfment at early CAD stages. However, it is probably more likely that low-grade inflammation associated with psychological distress and depression may reduce the stability of atherosclerotic plaques thereby increasing vulnerability towards plaque rupture and subsequent myocardial infarction and CHD-related death. The factors related to plaque rupture differ from those observed in gradual CAD progression because the typical duration of many psychological risk factors (e.g., depression, family and work-related distress) is not sufficiently long to initiate and sustain the gradual atherosclerotic process. Other psychosocial factors, such as personality traits and long-term effects of adverse early life exposures may be especially relevant to the early onset of atherosclerosis and its gradual progression. This hypothesis is consistent with the lack of consistent associations between depression and CAD severity at angiography. The dissociation between depression and CAD severity may be of particular relevance to the sex-specific differences in CAD and subsequent risk of myocardial infarction and CHD-related mortality. Estrogens may contribute to the differences in clinical phenotypes of IHD and the PNI mechanisms of disease development and progression.
Evidence in support of sex differences in inflammation markers that are relevant to IHD is accumulating. Sex differences have been observed in CRP levels predicting cardiovascular disease-related mortality. In the NHANES-III survey (Doran 2013) women displayed an increased prevalence of elevated (≥3 mg/L) CRP levels compared to men (32 % versus 20 %, respectively). This study also showed that elevated CRP levels (≥3 mg/L) were associated with cardiovascular mortality in men, but not in women after adjustment for risk factors (waist circumference, low HDL, presence of hypertension, diabetes, and elevated total cholesterol) (Doran et al. 2013). Increased circulating CRP levels have also been associated with risk factors for IHD, such as hypertension, diabetes, and obesity. In two cohort studies comparing women and men with and without diabetes, CRP levels were elevated and associated with coronary calcification in women, but not in men (Qasim et al. 2011). Levels of CRP were higher in women, compared to men, but only in persons who had metabolic syndrome, or components of metabolic syndrome (abdominal obesity, obesity, triglycerides, HDL, blood pressure) (Rudnicka et al. 2011).
Most studies on sex differences indicate higher levels of inflammation markers in women than men, but it is unclear whether the association between psychological factors with immune system markers differs between women versus men. Postmenopausal women with CAD who reported poor subjective sleep quality had higher CRP levels at baseline and increased levels of CRP, IL-6, and fibrinogen at 5-year follow-up compared to men (Prather et al. 2013). However, a meta-analysis of the relationship between depression and CRP has shown that this association was not significant in the 15 studies examining women (Howren et al. 2009). Moreover, the predictive value of inflammation markers for adverse cardiac health outcomes is less in women than in men and the association between depression and inflammation markers and depression is also less consistent in women compared to men. It is therefore possible that the role of inflammation in IHD is mediated by other factors in women versus men (e.g., abdominal obesity) (Arsenault et al. 2009). Because fluctuating levels of estrogens may adversely affect psychological wellbeing, the role of estrogens in the link between psychological factors, immune system-related disease processes, and IHD requires further investigation.
Estrogens and PNI Mechanisms of Atherosclerosis
Estrogens are steroid hormones derived from cholesterol and predominantly converted in the ovaries into estrone (E1), estradiol (E2), or estriol (E3). Estradiol is the dominant hormone during the reproductive years, and we will refer to this hormone as estrogen. Estrogen has different functions as it is involved in the reproductive cycle, fat deposition, HDL production, fluid balance, coagulation, and inflammation. Estrogen levels decline after menopause, and the number of estrogen receptors decrease with age. Estrogen influences many cells and systems because it directly affects intracellular processes when binding to its receptors. Estrogens can bind to three different receptors ERα, ERβ, both membrane and nuclear receptors, and GPR30, a G-protein coupled receptor, also known as G-protein coupled estrogen receptor-1 (GPER). It is estimated that the total number of genes activated or repressed by estrogen is 500 or more (Herrington and Howard 2003).
The fluctuating and declining estrogen levels in perimenopause and menopause, and the rise of atherosclerotic risk for women after menopause, make estrogen a plausible factor in atherosclerosis. Most relevant factors affected by estrogen for the process of atherosclerosis are circulating lipid levels and lipid oxidation, markers of inflammation, and endothelial dysfunction.
Estrogens and lipids: Estrogens have been found to lower LDL levels and increase HDL levels, thus improving the lipid profile in women during the premenopausal phase (Cossette et al. 2013; Knowlton and Lee 2012). Postmenopausal women show an increase in LDL and decrease in HDL levels. The shift in LDL/HDL is one of the atherogenic pathways affected by declining estrogen levels in menopause. These higher levels of circulating lipids may promote lipid deposition and combined with engulfment of T-cells and macrophages promote the development of foam cells and early atherosclerotic plaques.
Levels of circulating auto-antibodies against oxLDL have been found to correlate to the extent of atherosclerosis (Fairweather et al. 2012). The effect of estrogens on circulating auto-antibodies against oxLDL is a potential pathway that requires further investigation.
Estrogens and inflammation: Estrogens inhibit local production of pro-inflammatory markers such as CRP by decreasing IL-6 secretion (Cossette et al. 2013), whereas androgens have immunosuppressive properties associated with cell-mediated immunity. Consistent with these immune system-related effects of estrogens, postmenopausal women have increased circulating levels of pro-inflammatory cytokines IL-6 and TNF-α, and an increased response of the body to these cytokines (Gameiro 2010). Estrogens can inhibit the activity of adhesion molecules (e.g., reduced VCAM-1 expression), thereby reducing monocyte infiltration and subsequent inflammation of coronary arteries (Knowlton and Lee 2012). The estrogen receptor GPR30 is expressed in human endothelial cells, and it has been shown to partially attenuate TNF-induced upregulation of pro-inflammatory proteins such as ICAM-1 and VCAM-1 (Chakrabarti and Davidge 2012).
Estrogen also activates B-cells, part of the humoral immune response, thereby releasing antibodies against infection, vaccines or as part of autoimmune disorders (Fairweather et al. 2012). In contrast, androgens (testosterone, dehydroepiandrosterone, androsterone) reduce B-cell synthesis of antibodies, and have been found to increase Th1 cell-mediated immunity and inflammation (Fairweather et al. 2012). Postmenopausal women show a decrease in B lymphocytes and B-cell activating CD4 T-helper cells, and a subsequent increased susceptibility to infection (Gameiro et al. 2010). This profile is consistent with the increased prevalence for autoimmune disorders in women.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


