Prosthetic Valvular Disease
There are two major classes of prosthetic valves, mechanical and bioprosthetic (Fig. 36.1). Each specific valve within these groups has unique features that provide differences in hemodynamics, durability, and thromboembolic risk.
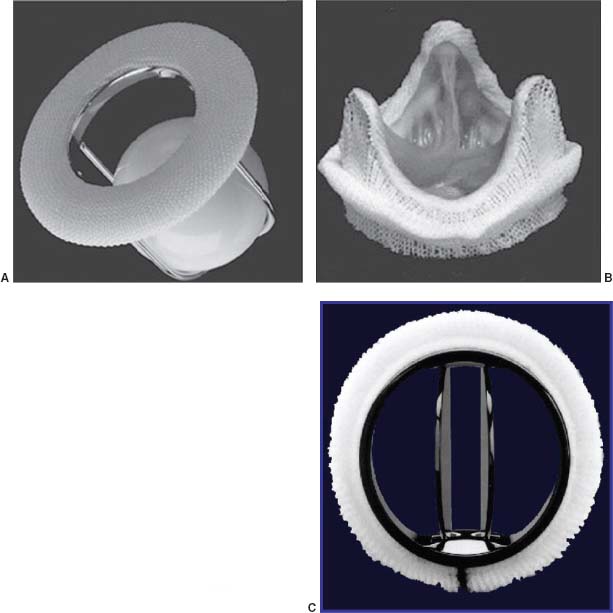
FIGURE 36.1 A:Ball-in-cage valve. B:Bioprosthetic valve. C:Bileaflet mechanical valve.
MECHANICAL VALVES
The three main types of mechanical valves available are bileaflet, tilting disc, and ball-in-cage. Bileaflet tilting disc valves include the St. Jude’s Medical (see Fig. 36.1A), Carbomedics, and On-X valves. The symmetric flow across the bileaflet system provides excellent hemodynamics, with lower transvalvular pressure gradients at any outer diameter or cardiac output than the tilting disc valves or ball-in-cage types. Along with lower rates of mechanical failure or thromboembolism, bileaflet valves are currently the most commonly used mechanical prostheses worldwide.
Single tilting disc valves such as the Bjork–Shiley (see Fig. 36.1B) or Medtronic Hall valves consist of a metallic sewing ring attached to a tilting disc that rotates about an off-centered pivot axis. Some models of the convexoconcave Bjork–Shiley valve demonstrated a high degree of strut fracture (2% per year) and embolization of the disc, resulting in withdrawal of these valves from the market, and prophylactic valve replacement in those whose risk of embolization exceeded the risk of reoperation. Valves at particular risk for this are larger valves in the mitral position implanted in younger patients. Since no Bjork–Shiley valve has been implanted in more than 20 years, the at-risk population is getting quite small. The earlier tilting disc valves also have greater thrombosis risk than newer Medtronic-Hall valves, in which a small orifice in the center permits regurgitant flow to “wash” potentially thrombogenic material from the disc.
The Starr–Edwards valve (see Fig. 36.1C) is the prototypical ball-in-cage valve. Ball-in-cage valves demonstrate a less favorable hemodynamic profile and a higher incidence of thromboembolic complications. Despite these limitations, however, some Starr–Edwards valves have performed for over 40 years.
BIOPROSTHETIC VALVES
Bioprosthetic valves fall into one of two categories: heterografts, such as Carpentier–Edwards (CE, see Fig. 36.1D), which are non–human tissue valves, and homografts, which are cadaveric human aortic valves within a small portion of the donor’s aortic root for support. Compared to mechanical valves, bioprosthetic valves require less anticoagulation, but they are less durable. Stented heterografts are manufactured from porcine valvular tissue (e.g., Medtronic Mosaic valve) or bovine pericardium (e.g., CE Perimount valve). With improvements in design and preseration techniques, currently produced bovine and porcine valves are expected to have comparable durability. Aortic homografts are harvested from cadaveric hearts and cryopreserved. They may be implanted as isolated valves in the subcoronary position or, more commonly, with a short segment of the donor’s aortic root, in which the recipient’s coronary arteries are reimplanted. Although the durability of homograft prostheses may be slightly higher, they are less frequently used due to the complexity of reoperation (calcification of the root and need for reimplantation of the coronary arteries). Homografts have a reduced rate of early reinfection and are the valve of choice in aortic valve endocarditis (Fig. 36.2).
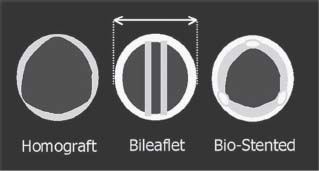
FIGURE 36.2 Although mechanical and bioprosthetic valves have similar external diameters, the figure demonstrates that the stented bioprosthetic valves have a smaller internal diameter and thus a more unfavorable hemodynamic profile.
SELECTION OF VALVE TYPE
Valve repair is preferred over replacement, when feasible. Mitral valves are much more frequently repaired, with repair offering advantages of preserving left ventricular (LV) function via conservation of the subvalvular apparatus, lower operative mortality, higher long-term survival rate, and freedom from anticoagulation. Aortic valve repair is less frequent, although possible in cases with predominant regurgitation due to prolapse or redundancy without severe stenosis or calcification.
Multiple factors need to be considered in selecting a prosthetic valve, including the age of the patient, the probability of future pregnancy, life expectancy, occupation, and lifestyle. Mechanical valves are more durable than bioprosthetic valves, but they require a commitment to chronic anticoagulation. Age recommendations differ with valve position, as bioprosthetic mitral valves deteriorate more rapidly than aortic valves. Mechanical aortic and mitral valves are generally recommended for patients younger than 60 and 65 years of age, respectively, who have no contraindications to anticoagulation and are expected to be medically compliant. The minimum age for a bioprosthesis is later for mitral than for aortic valves due to the more rapid deterioration of bioprostheses in the mitral position. The most recent valve guidelines, however, emphasize patient choice, particularly lifestyle, in valve implantation. With reduction in the risk of redo valve surgery, many patients 50 years of age or younger are opting for bioprosthetic valves.
The development of transcatheter aortic valve replacement (TAVR) is encouraging a further move toward bioprosthetic valves for younger patients: one may choose the benefits of less anticoagulation now in spite of the decreased valve durability, banking on the hope that future TAVR will carry a lower procedural risk should future valve replacement be required.
ANTICOAGULATION
Anticoagulation is a frequent topic of cardiology consultation and board testing. We review here the essentials including general guidelines for mechanical and bioprosthetic valves, therapy adjustment after thromboembolic events, perioperative management, and management in pregnancy.
1. Anticoagulation for mechanical prosthetic valves (Table 36.1). For board review purposes, recommendations are simplified here to just the Class I recommendations, except where indicated. Aortic position bileaflet and Medtronic-Hall tilting disc valves, without risk factors, may be anticoagulated to an international normalizing ratio (INR) of 2.0 to 3.0. All other aortic mechanical valves and all mitral mechanical valves should be anticoagulated to an INR of 2.5 to 3.5. Furthermore, patients with even low-risk aortic mechanical valves and any thromboembolic risk factors, such as atrial fibrillation, previous thromboembolism, hypercoagulable state, or severe systolic dysfunction (left ventricular ejection fraction [LVEF] <30%) should also be anticoagulated to 2.5 to 3.5. All patients with prosthetic valves should be on aspirin (81 mg daily).
Rates of thromboembolism are highest with Starr–Edwards valves, followed by single tilting disc valves. Bileaflet tilting disc valves have the lowest reported rates of thromboembolism, because the built-in regurgitation acts to “clean” debris off the valve. Regardless of the type of valve, at appropriate levels of anticoagulation, the incidence of thromboembolism is <1% in those maintained on therapeutic anticoagulation. The majority of patients who experience thromboembolic complications have subtherapeutic INR at the time of the event. Currently, there is no approved use of dabigatran in anticoagulation of prosthetic heart valves (PHVs), though future trials may lead to expansion of the indication to include this.
TABLE
36.1 Recommended Anticoagulation Therapy for Patients with Prosthetic Valves
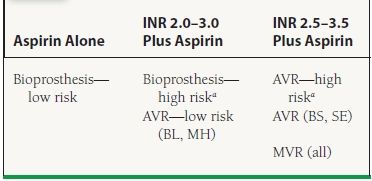
INR, international normalizing ratio; AVR, aortic valve replacement; MVR, mitral valve replacement; BL, bileaflet; MH, Medtronic-Hall; BS, Bjork–Shiley; SE, Starr–Edwards.
aHigh Risk defined as atrial fibrillation, prior thromboembolism, hypercoagulable state, or severe left ventricular dysfunction (EF < 30%). From Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2008;52:e1-e142, with permission from Elsevier.
2. Anticoagulation for bioprosthetic valves. Patients with bioprosthetic aortic or mitral valves and no risk factors may be maintained on aspirin alone. Patients with risk factors should be anticoagulated to an INR of 2 to 3. As with mechanical valves, risk factors are atrial fibrillation, prior thromboembolic event, hypercoagulable state, or severe LV dysfunction (EF < 30%).
Early postoperative anticoagulation: In the early postoperative period, the approach to anticoagulation for bioprostheses varies widely. American College of Cardiology/American Heart Association (ACC/AHA) guidelines give a class Ila recommendation to warfarin starting 2 to 4 days following surgery, after epicardial wires are removed. Bioprosthetic valve recipients without risk factors may discontinue warfarin after 3 months and continue aspirin alone. In many center, however, low-risk patients are managed entirely without warfarin.
3. Adjustment of anticoagulation after a thromboembolic event. Patients who have an embolic event while therapeutically anticoagulated should have their therapy increased as follows:
 On Warfarin, not taking aspirin: add aspirin 81 mg daily
On Warfarin, not taking aspirin: add aspirin 81 mg daily
 INR 2 to 3 and aspirin: Increase INR to 2.5 to 3.5.
INR 2 to 3 and aspirin: Increase INR to 2.5 to 3.5.
 INR 2.5 to 3.5 and aspirin: Increase INR to 3.5 to 4.5.
INR 2.5 to 3.5 and aspirin: Increase INR to 3.5 to 4.5.
 Aspirin alone: Add Warfarin to target INR 2 to 3. Guidelines also give options to increase dose to 325 mg daily or add clopidogrel 75 mg daily.
Aspirin alone: Add Warfarin to target INR 2 to 3. Guidelines also give options to increase dose to 325 mg daily or add clopidogrel 75 mg daily.
4. Perioperative anticoagulation for noncardiac surgery. For patients with mechanical valves who require major surgery with anticipated substantial blood loss, warfarin should be stopped 2 to 3 days prior to the procedure, to achieve an INR level of 1.5 or less, and restarted 24 hours after the surgery. For low-risk patients with bileaflet aortic valve (i.e., baseline target INR of 2 to 3), the short-term risk is so low that routine heparinization is not recommended. For all patients with a target INR of 2.5 to 3.5 (any mitral mechanical valve, aortic mechanical valves excluding bileaflet or Medtronic-Hall, or any risk factors—see Table 36.1), hospital admission is recommended with initiation of heparin when INR falls below 2.0. Postoperatively, heparin should be restarted as soon as it is considered safe and continued until therapeutic anticoagulation is achieved with warfarin. For minor procedures, in which blood loss is minimal, anticoagulation can be continued. High-dose vitamin K should not be given routinely (class III recommendation), as this may create a hypercoagulable state. Use of fresh frozen plasma is preferable in emergency situations.
5. Pregnancy. Warfarin is teratogenic between 6 and 12 weeks of gestation. Women requiring warfarin therapy who are attempting to become pregnant should have frequent pregnancy tests and cease warfarin when pregnancy is achieved. They should then use dose-adjusted subcutaneous unfractionated heparin (UFH), continuous IV UFH, or low molecular weight heparin (LMWH) for the remainder of the first trimester. Subcutaneous heparin, dosed 17,500 to 20,000 units twice daily, should be adjusted to a target activated partial thromboplastin time (aPTT) of at least twice the control, checked 6 hours after injection. LMWH is dosed twice daily, adjusted to maintain an anti-Xa level between 0.7 and 1.2 units per mL, checked 4 hours after administration. LMWH should only be used if anti-Xa levels are appropriately monitored.
Patients may resume warfarin therapy for the second and third trimesters. As pregnancy is a hypercoagulable state, all pregnant women with mechanical valves on warfarin should be treated to a target INR of 2.5 to 3.5. In those patients who resume warfarin, it should again be discontinued 2 to 3 weeks before term and continuous UFH initiated. Low-dose aspirin can be used in conjunction with anticoagulation therapy in the second and third trimesters. Nursing mothers can safely use both heparin and warfarin, which do not appear to be secreted into breast milk.
COMPLICATIONS OF VALVE PROSTHESES
Monitoring By Echocardiography
A postoperative transthoracic echocardiogram (TTE) should be obtained either prior to discharge or within 4 weeks of discharge. Asymptomatic uncomplicated patients should follow-up annually, although routine TTEs are not indicated in the absence of a change in clinical status. In asymptomatic patients with mechanical valves, no further follow-up echocardiography is required, in the absence of other indications. In patients with bioprosthetic valves, annual TTEs may be considered after the first 5 years. Of course, a TTE is indicated in any patient with a prosthetic valve whenever there is a change in clinical status, new murmur, question of valve function, or concerns of ventricular function. Of note, cardiac magnetic resonance imaging (MRI) is safe for all available prosthetic valves, though artifact induced by metal in the valve will obscure adjacent structures.
Normally functioning mechanical and bioprosthetic valves all have gradients across them, with mean gradients up to 14 mm Hg in the aortic position (except Starr–Edwards, up to 24 mm Hg) and up to 7 mm Hg in the mitral position. Conditions with increased cardiac output such as anemia, tachycardia, pregnancy, hyperthyroidism, or severe prosthetic, leak can lead to higher-than-normal gradients and give the false impression of prosthetic stenosis. Occasionally, a high gradient across an aortic valve demonstrates a prosthesis–patient mismatch, which is defined as <0.6 cm2/m2. The recently published prosthetic valve guidelines from the American Society of Echocardiography provide comprehensive normal values for all prosthetic valves in common use.
Pressure recovery can complicate measurements across bileaflet mechanical prostheses (Fig. 36.3). In this situation, flow deceleration through the gently flaring central orifice may cause Doppler to overestimate the true pressure gradient by up to one-third. This phenomenon may be particularly problematic with small mechanical bileaflet prostheses in the aortic position. Given the need to follow transvalvular gradients to exclude pannus, thrombus, or stenosis secondary to increasing calcification, it is critical to obtain a baseline echocardiogram early postoperatively for future reference.
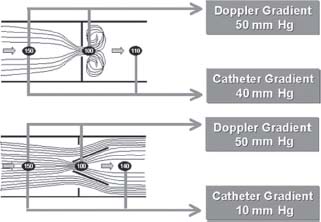
FIGURE 36.3 Demonstration of pressure recovery. The higher pressure gradient recorded through a prosthesis by Doppler overestimates the true pressure gradient as a result of flow acceleration through a narrowed orifice. Pressure recovers distally, at the position of the catheter recording. This occurs primarily with small mechanical bileaflet prostheses in the aortic position.



