I. INDICATIONS FOR IMPLANTATION
A. Types of prosthetic valves.
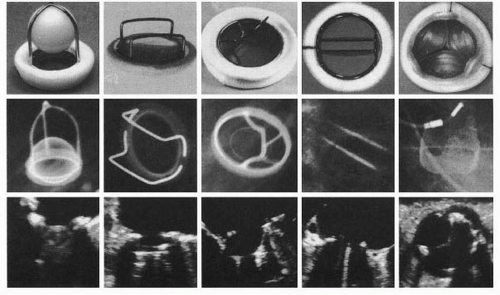
Prosthetic valves are classified into two major categories: mechanical and bioprosthetic. Each model differs in its durability, thrombogenicity, and hemodynamic performance. Various mechanical and bioprosthetic valves are shown in
Figure 18.1.
1. Bioprosthetic valves.
These resemble native valves but have a slightly less optimal hemodynamic performance, owing to the reduction in flow profile by interposed stents and the sewing ring.
a. Heterografts
(1) Carpentier-Edwards valves are made of either bovine pericardium (aortic position), which have greater durability, or porcine leaflets mounted on a cloth-covered annular ring and supported by steel alloy flexible stents at each of the commissures.
(2) The Hancock II stented porcine valve (Medtronic) has been considered as the porcine bioprosthetic valve with the best hemodynamics and longevity available. The supraannular prosthetic sewing ring improves hemodynamic performance; and modern preservation techniques using low-pressure fixation and treatment with sodium dodecyl sulfate increase longevity by delaying calcification. The durability of bioprosthetic bovine pericardial versus porcine valves is controversial, although many consider that the pericardial valves may have some durability advantage in younger patients.
(3) For the stentless porcine bioprostheses (Medtronic Freestyle or St. Jude Medical), there are three different methods for implantation, with the subcoronary valve replacement being the most common. Although the stentless valves offer a better hemodynamic profile owing to the larger effective orifice area (EOA), convincing advantages in terms of mortality, left ventricular (LV) mass regression, and durability (when compared with pericardial and not porcine valves) have yet to be demonstrated.
(4) Since the first-in-man
TAVR done by Cribier in 2002, more than 40,000 patients have undergone TAVR worldwide (see
Chapter 66). The technology has evolved tremendously and is now transforming the management of patients with critical aortic stenosis who are high-risk surgical candidates. The ground-breaking Placement of AoRtic TraNscathetER Valves (PARTNER) trial changed the paradigm. It randomly assigned
patients (
n = 358) with severe aortic stenosis, whom surgeons considered not to be suitable candidates for surgery, to standard therapy (including balloon aortic valvuloplasty) or transfemoral transcatheter implantation of a balloon-expandable bovine pericardial valve. The primary end point was the rate of death from any cause.
The results showed that this approach was associated with a significant reduced 1-year mortality for patients with severe aortic stenosis who are not surgical candidates due to advanced comorbidities (50.7% for medical treatment vs. 30.7% in the TAVR group; hazard ratio with TAVR, 0.55; 95% confidence interval [CI], 0.40 to 0.74;
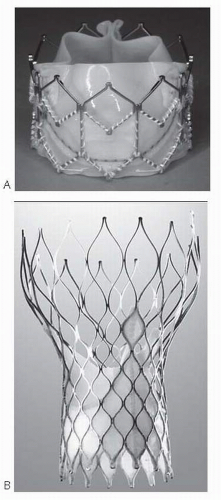
p < 0.001). The valve tested was Edwards SAPIEN heart valve system (Edwards Lifesciences) that consisted of a trileaflet bovine pericardial valve and a balloon-expandable, stainless steel support frame (
Fig. 18.2).
The second largest experience, mostly European, is with the selfexpanding Medtronic Core Valve ReValving system. It uses a porcine pericardial valve in a larger and self-expandable nitinol frame, which covers both the left ventricular outflow tract (LVOT) and the aortic root. It has also demonstrated similar trends in outcome, although with slightly higher incidence (up to 25%) of atrioventricular block requiring pacemaker implantation.
The long-term durability of these valves has been addressed only in small studies. Theoretically, and according to the manufacturer’s wear test, both transcatheter valve systems are designed to last ≥10 years. All published studies suggest good durability and preserved hemodynamic function, with EOAs over 1.5 cm2 at 3 years. Paravalvular leak has been a concern with these valves, but this is generally mild and does not usually progress significantly at least over medium-term follow-up.
b. Aortic homografts are cryopreserved cadaveric human aortic valves. These are typically implanted stentless, with a short segment of the donor’s aortic root for support. The coronary arteries require reimplantation. The hemodynamic profile of the homograft is similar to that of the native valve. Availability of homografts is a limiting factor.
c. Autograft. An autograft is a procedure in which the patient’s own valve is moved from its normal anatomical position to another site. Typically, this is done with the pulmonary valve in patients with significant aortic valve disease. A pulmonary homograft is placed at the native pulmonary position. This operation is called the Ross procedure, after the surgeon who popularized it. This procedure has the advantage of putting a native valve at the hemodynamically most important position. It has been advocated for younger patients, and some reports suggest that the autograft may grow with the patient, which is advantageous in the adolescent age group. However,
the initial enthusiasm with this procedure has been tempered by suboptimal outcomes in many adult patients, particularly with regard to the pulmonary homograft. Additionally, progressive root enlargement may ensue in patients with bicuspid valve and ascending aortic dilatation, leading to autograft failure. The decision to proceed to autograft implantation in adults should be considered very carefully and in consultation with a surgeon with extensive experience of this procedure.
2. Mechanical valves
a. Single-leaflet tilting disk. This valve (e.g., Björk-Shiley, Medtronic-Hall, and Omniscience) consists of a metallic sewing ring attached to a tilting disk made of pyrolytic carbon that rotates about an off-centered pivot axis, with a range of about 60° to 85° from the occluded to the open position. When open, the prosthesis has two orifices separated by the occluder. The major orifice is formed as the disk swings downstream to the open position. The disk on the other side of the pivot axis swings proximally, forming the minor orifice.
b. Bileaflet tilting disk. The St. Jude and CarboMedics valves have two semicircular pyrolytic carbon disks that rotate freely through 75° to 90°. Two large lateral orifices and a small central rectangular space are created in the open position. A built-in leakage volume is designed to reduce thrombus formation on disks.
c. Caged ball. The Starr-Edwards valve consists of a silicone ball within a cage attached to a metallic alloy ring. The ball is free to travel along the cage over a distance of 1 to 2 cm. Flow across the prosthesis is directed circumferentially around the ball. The hemodynamic profile is less favorable than that of the tilting disk prosthesis. This is the valve with the greatest durability, with a 30-year follow-up in some studies.
B. Selection of valves.
Table 18.1 summarizes the clinical factors that favor selection of a bioprosthetic versus a mechanical valve. The choice is largely dependent upon the age of the patient at the time of prosthetic valve implantation and on which complication the patient wants to avoid: specifically, anticoagulation therapy and its complications with the mechanical valve and structural valve deterioration with a bioprosthesis.
The recommendation to use tissue valves in older patients and mechanical valves in younger patients is based on information obtained from older trials. There have
been no randomized controlled trials after 1982 comparing mechanical with bioprosthetic valves, which makes the decision difficult, as newer bioprosthetic valves may be more durable than older ones. There has been a shift toward using bioprosthetic valves in younger patients over the last decade.
1. Valve repair. The feasibility of native valve repair instead of replacement should always be considered prior to surgery (
Table 18.2). Currently, the greatest experience is with mitral valve repair. If feasible, mitral valve repair offers several potential advantages over replacement, including
preservation of LV function via conservation of the subvalvular apparatus, lower operative mortality, higher long-term survival rate, and freedom from anticoagulation. Mitral valve repair may be considered for asymptomatic patients with severe mitral regurgitation if there is a high chance of repair at high-volume centers.
An aortic valve with predominant regurgitation due to prolapse, but without severe stenosis or calcification, can also be repaired. Intraoperative transesophageal echocardiography (TEE) appears to have a role determining the quality of the repair and predicting the long-term durability.
2. Bioprosthetic valves are indicated in patients with
a contraindication to chronic anticoagulation and are preferred for
patients ≥65 years (70 years in the mitral position) due to reasonable durability, favorable hemodynamic profile, and freedom from chronic anticoagulation. Approximately 30% of heterograft bioprostheses fail within 10 to 15 years of implantation, although the incidence of bioprosthesis failure is age dependent (
Table 18.3). Overall complication rates for aortic bioprosthetic and mechanical valves are similar at 12 years, with a higher rate of reoperation for bioprosthetic valves and a higher rate of hemorrhage with mechanical valves. The advent of newer low-profile bioprostheses and the apparent improved durability of later models have led to an increase in their use, especially in patients who wish to avoid anticoagulation.
3. Transcatheter aortic valve replacement, as mentioned before, is now indicated in patients with severe aortic stenosis who are considered inoperable or high risk for conventional open heart surgery due to advanced comorbidities. A comprehensive evaluation for procedural eligibility and candidacy is required including coronary angiography to exclude significant coronary artery disease. If significant coronary lesions are present, they should be revascularized percutaneously and the TAVR procedure is usually deferred for ≥1 month. Computed tomography (CT) angiography with three-dimensional vessel reconstruction is also required to determine the suitability of the iliofemoral access. Dedicated imaging, in particular of the infrarenal aortic segment to the femoral arteries, is needed for sizing of the arterial access (preferably > 6 mm in diameter), vessel tortuosity, and calcification of the iliac arteries. Lastly, TEE, both preprocedural and intraprocedural,
is important in the evaluation of the aortic annulus size, aortic valve morphology, and calcification of the aortic root and aortic valve leaflets. It can enhance procedural success in determining appropriate valve and device sizing selection in addition to continuous monitoring for procedural complications.
The transapical approach requires a small left thoracotomy to allow direct puncture and sheath introduction into the left ventricle for antegrade delivery of the TAVR system. Patients who require this approach have a higher incidence of peripheral vascular disease, which is a marker of worse long-term outcome. Nonetheless, in patients who require AVR but have aortic atheroma or peripheral vascular disease that limits their candidacy for retrograde transcatheter AVR, the transapical approach should be considered.
4. Homografts. The homograft is the valve of choice in aortic valve endocarditis and has the lowest valvular gradient among the bioprosthetic valves. Durability was thought to be superior to that of heterografts, but recent studies throw some doubt on this. Only 10% are still functioning after 20 years. The primary operation is more difficult with homografts, as the coronary arteries require implantation. Reoperation is also more complex, as the homograft frequently calcifies and is difficult to remove and replace. The main current indication for an aortic homograft is complex endocarditis involving a native valve, especially in prosthetic valve endocarditis and abscess where the risk of reinfection of a new prosthesis is high. Another indication is in older patients with small aortic root and LVOT in order to maximize hemodynamics and minimize the transaortic gradient.
5. Mechanical valves. Mechanical valves are
more durable than bioprosthetic valves; some can last > 20 years. Mechanical prostheses are generally recommended for
patients <
50 years because of greater durability and for
patients already on permanent anticoagulation for previous stroke or arrhythmia. The stroke risk of about 1% per annum for patients with a mechanical valve receiving appropriate anticoagulation management is similar to that for a bioprosthetic valve without anticoagulation. In
younger patients requiring combined aortic and mitral valve replacement, mechanical valves are preferred, given the more rapid rate of prosthesis deterioration in the mitral position.
Pregnancy should be discouraged in patients with mechanical prostheses because of the high risk to the mother and the fetus (see
Chapter 38). Given their lower profile, mechanical prostheses may be preferred in
patients with small ventricles. Issues of
compliance with anticoagulation and risks of trauma should be integrated into the selection of a mechanical valve.
a. St. Jude Medical and Medtronic-Hall valves are the most popular prosthetic valves because of their favorable hemodynamic performance, longevity, and low rates of complications. Loss of structural integrity has been reported in a small percentage of patients with St. Jude valves, whereas the primary concern with the Medtronic-Hall valve is the potential for occluder impingement during placement.
b. Starr-Edwards valves are the most durable of all the prosthetic valves. However, they are less popular today because of their thrombogenicity and suboptimal hemodynamic performance in comparison with tilting disk valves.
c. Manufacture of the Björk-Shiley valve was discontinued in 1986 following published reports of complications with strut fracture.
6. The decision with regard to the optimal valve type should be made in consultation with the cardiothoracic surgeon and patient and should not solely be based on age. Lifestyle, compliance, other medical issues, and consideration of pregnancy all have an impact on the decision.