Fig. 13.1
Survival among the 41 patients treated with epoprostenol and the 40 patients receiving conventional therapy. Data on patients who underwent transplantation during the 12-week study were censored at the time of transplantation. Estimates were made by the Kaplan–Meier product-limit method. The two-sided p value from the log-rank test was 0.003. Survival analysis with data on patients receiving transplants not censored at transplantation resulted in the same level of significance (two-sided p = 0.003 by the log-rank test). From [33]
A second prospective randomized controlled trial of epoprostenol was conducted in patients with PAH associated with scleroderma [34]. One hundred and twenty-one symptomatic patients in NYHA functional class 3 or 4 were randomized 1:1 to epoprostenol versus standard of care. At the end of the 12-week period, patients randomized to epoprostenol were receiving an average of 11.2 ng/kg/min of epoprostenol. The main outcome, 6MWD, improved from a median of 270 to 316 m in the treated group, while the group assigned to standard of care declined from a median of 240 to 192 m (p < 0.001). Similarly, significant improvements in functional class and pulmonary hemodynamics were noted in the treated arm, while mortality was not different.
Initial Dose and Titration Strategy
Epoprostenol continuous infusion is usually initiated at a dose of 2 ng/kg/min and the dose is gradually uptitrated over the next several months. Although the frequency of titration and the average dose used varies from center to center and from country to country, the general rule is to uptitrate once or twice a week by a few ng/kg/min in the first month, until side effects occur. Thereafter, uptitration is performed at a slower pace over the following months to improve exercise capacity, functional class, and hemodynamics, while minimizing side effects. The side effect profile of epoprostenol is usually well tolerated in most subjects. These include flushing, headache, nausea, loose stools, jaw discomfort with “first bite,” and foot pain with prolonged standing or walking (Table 13.1) [35–37].
Table 13.1
Side effects and complications of parenteral and inhaled prostacyclins
Route of administration | |
|---|---|
Side effects | |
Headache | Intravenous, subcutaneous, inhaled |
Jaw pain with first bite | Intravenous, subcutaneous, inhaled |
Nausea, vomiting | Intravenous, subcutaneous, inhaled |
Diarrhea | Intravenous, subcutaneous, inhaled |
Leg pain | Intravenous, subcutaneous, inhaled |
Flushing, rash | Intravenous, subcutaneous, inhaled |
Restlessness | Intravenous, subcutaneous, inhaled |
Cough | Inhaled |
Site pain | Subcutaneous |
Complications | |
Line infection | Intravenous |
Bloodstream infection, sepsis | Intravenous |
Catheter breakage, occlusion | Intravenous, subcutaneous |
Pump malfunction | Intravenous, subcutaneous |
Hemorrhage from the site | Intravenous, subcutaneous |
Syncope | Intravenous, subcutaneous, inhaled |
Paradoxical embolism | Intravenous |
Thrombocytopenia | Intravenous, subcutaneous |
Experience with Epoprostenol Infusion in PAH
In an open-label trial of 18 patients with IPAH, Kaplan–Meier estimates of 1-, 2- and 3-year survival were 86.9 %, 72.4 %, and 63.3 %, respectively, compared with 77.4 %, 51.6 %, and 40.6 % observed prior to the development of epoprostenol [38]. Cohort analyses in Europe and in the United States have provided convincing evidence of the long-term benefits of epoprostenol infusion. One year of epoprostenol therapy in 162 IPAH patients from France was associated with improvements in clinical function and hemodynamics [35]. Similarly, 178 IPAH patients treated in the US had improved survival compared with that observed in the NIH registry which was conducted before epoprostenol was developed and used as a historical control [36]. The survival rate at 1 year was 85 % vs. 58 %, 2 years 70 % vs. 43 %, 3 years 63 % vs. 33 %, and at 5 years 55 % vs. 28 % (p < 0.001). Adverse prognostic factors included a clinical history of right heart failure, advanced functional class, and hemodynamic parameters such as an elevated right atrial pressure and depressed cardiac index.
Long-term administration of PG requires a permanent central venous catheter and a portable infusion pump [37]. The complex delivery system requires education regarding sterile technique, operation of the pump, and care of the catheter. The original formulation of epoprostenol (Flolan®) needs to be prepared daily and requires that the drug cassette be kept cool with ice packs due to limited stability of the drug at room temperature (approximately 8 h at room temperature). A strong support system, and a “partner” who is willing to learn how to mix and deliver the drug is strongly recommended to obviate problems if the subject is too ill or unable to prepare medication on a given day. Serious complications include infection (exit site and/or bacteremia) thrombosis of the catheter, and risk of temporary interruption of the infusion because of iatrogenic “switching” or pump malfunction (Table 13.1) [39]. The incidence of catheter-related sepsis ranges from 0.1 to 0.6 cases per patient-year [26, 39].
Evidence also supports epoprostenol use in PAH from associated etiologies. Similar improvements in exercise functional capacity and functional class have been seen in subjects with PAH associated with congenital left-to-right cardiac shunts [40], portal hypertension [41], and human immunodeficiency virus (HIV) infection [42].
Generic epoprostenol sodium was approved by the FDA in April 2008 as a therapeutic equivalent to branded epoprostenol (Flolan®), based on pharmacologic bioequivalence. Generic epoprostenol was available in the US for a brief period of time after its approval, although its production was interrupted due to manufacturer-related problems. In addition to generic epoprostenol, FDA approved epoprostenol-ES (Veletri®) in 2010, a new epoprostenol formulation that has greater drug stability at room temperature. After reconstituting as directed, epoprostenol-ES is stable for up to 5 days when refrigerated (36–46 F; 2–8 °C), or up to 48 h at room temperature (77 F; 25 °C), which makes the use of frozen gel packs unnecessary and allows more drug to be prepared in advance for convenient storage [43]. Recent experience supports the safety and efficacy of epoprostenol-ES as new therapy and as a transition from epoprostenol [44, 45].
Parenteral Prostacyclin Analogs
Treprostinil is a tricyclic benzidene prostacyclin analog that shares pharmacologic actions similar to epoprostenol, including antiproliferative activity on human pulmonary arterial smooth muscle cells [46]. Treprostinil differs from epoprostenol in that it is highly chemically stable at room temperature and neutral pH and has a longer half-life (3–4 h) [47]. The improved stability of this compound and its solubility at physiologic pH enables subcutaneous infusion, thereby avoiding the potential complications of the intravenous delivery system used for epoprostenol administration. The bioavailability of subcutaneous treprostinil is excellent, as demonstrated in healthy volunteers [47].
Subcutaneous treprostinil was approved in 2002 for the treatment of PAH patients of NYHA class II–IV. A 12-week multicenter, randomized, double-blind trial that enrolled patients with idiopathic PAH, PAH related to congenital heart defects or connective tissue disease, compared treprostinil to placebo in 470 patients [48]. Treprostinil significantly improved the main outcome, 6MWD, as well as dyspnea, fatigue, and signs and symptoms of pulmonary hypertension. At 12 weeks, there was also an improvement in hemodynamic parameters, including right atrial pressure, mean PAP, PVR and cardiac output. Although the 6MWD improvement in the treatment arm was minimal (a median difference of 16 m, compared with the placebo group), there was a clear dose–response relationship, with patients receiving more than 13.8 ng/kg/min increasing their walk distance by 36 m, while those receiving less than 10 ng/kg/min showing no improvement (Fig. 13.2). In a subset analysis from the subcutaneous trials, patients who had PAH related to connective tissue disease also experienced improvement in exercise capacity, symptoms of PAH, and pulmonary hemodynamics [49].
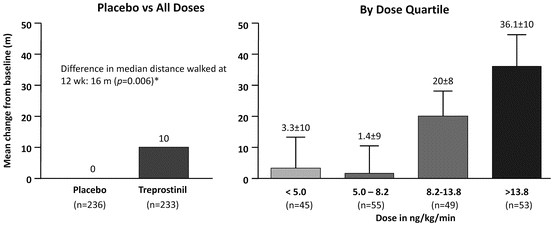

Fig. 13.2
Mean change in the 6-min walk distance from baseline to week 12 versus week 12 treprostinil dose quartile. From [48]
Subcutaneous infusion of treprostinil does not require daily mixing and preparation of the infusion. The drug comes premixed and the pump reservoir holds enough drug for approximately 72 h of infusion. There is no need for ice packs, or intravenous catheter care. The infusion site can be changed every 3–4 days, although some patients use the same site for 3–6 weeks, as long as the site is clean and pain at the site of infusion is tolerable. The most common side effect is pain at the site of infusion, which responds usually to a combination of local anesthetic solutions, nonsteroidal anti-inflammatories, gabapentins, or low-dose narcotics (Table 13.1) [50]. Usually the pain subsides within 48 h after the start of a new site. The most common site of infusion is the abdomen, although some patients prefer the flank, thigh, or shoulder area.
The FDA has also approved the use of intravenous treprostinil based on bioequivalence to its subcutaneous formulation [47]. The advantage over intravenous epoprostenol is that the cassette is changed every other day and, unlike the original form of epoprostenol (Flolan) but same as the newer form (Veletri) it does not require ice packs. The longer half-life of intravenous treprostinil may also decrease the risk of cardiovascular collapse in case of inadvertent temporary interruption of the infusion. In a prospective, open-label study, 31 PAH patients stable on intravenous epoprostenol were transitioned to intravenous treprostinil [51]. Twenty-seven patients completed the 12-week protocol and four patients transitioned back to epoprostenol. Treprostinil was dosed to minimize side effects, while avoiding symptom worsening. Overall, patients maintained their walks and functional class, although pulmonary hemodynamics worsened slightly, suggesting that treprostinil is an alternative to intravenous epoprostenol in selected patients who are being followed very closely. It is important to note that treprostinil is less potent than epoprostenol on a per ng basis, so higher doses may be required. Long-term experience with intravenous treprostinil was reported in a 48-week prospective, multicenter, open-label trial in 16 PAH patients on no prior PAH-specific therapy and in 31 patients transitioned from intravenous epoprostenol [52]. In de novo patients, intravenous treprostinil increased the 6MWD by a mean of 125 m and improved secondary endpoints, while 23 of the transitioned patients maintained their walks and functional class. Five patients died during the trial of causes not related to the therapy and seven discontinued due to adverse events. Lastly, a 12-week multicenter, randomized, double-blind, placebo-controlled trial was conducted in India [53]. Forty-four patients were enrolled before the study was terminated early due to safety concerns. A high number of adverse events related to catheter placement and sepsis were seen in both the placebo and treprostinil treatment arms. The end of the study analysis showed that treprostinil increased 6MWD by a placebo-corrected median of 83 m (p = 0.008) and improved functional class and dyspnea score.
As intravenous treprostinil requires the same delivery system as epoprostenol, concerns over catheter-related complications are also present. An early report to Center of Disease Control and Prevention showed higher bloodstream infections with treprostinil compared with epoprostenol, especially with gram-negative water-born bacteria [54]. Subsequent modifications in the delivery system to a closed hub system, as well as using Flolan diluent that has a higher pH than the normal saline diluent designed for use with treprostinil, showed a decrease in the risk of bloodstream infections [55, 56].
Inhaled Prostacyclins
The rationale of using the inhaled route for prostacyclin delivery came from the notion that local distribution would avoid systemic side effects and complications associated with parenteral administration. Iloprost is a prostacyclin analog that has been used intravenously in some countries in Europe. Early trials demonstrated acute and chronic (3 months) pulmonary vasodilator properties of iloprost [57]. Its acute effect lasted 90 min. Subsequently, the pivotal randomized placebo-controlled trial of inhaled iloprost enrolled 203 patients with IPAH, PAH associated with connective tissue disease, drugs and toxins, or chronic thromboembolic disease in NYHA functional class 3 and 4 [58]. Patients averaged 7.5 inhalation treatments a day. When compared with the placebo arm, significantly more patients receiving iloprost reached the primary endpoint, defined as the combination of functional class improvement and increase in 6MWD of at least 10 % in the absence of deterioration or death. In addition, significant improvements were recorded in dyspnea and quality of life scores, as well as in post-inhalational hemodynamic parameters (Fig. 13.3). There was a trend toward more syncopal events in the treatment arm. Subsequently, long-term experience with inhaled iloprost was reported in 24 patients treated for at least 1 year [59]. Nevertheless, the limitations of efficacy of inhaled iloprost when compared with parenteral prostacyclins were described in selected patients who deteriorated on the inhaled therapy and were rescued by transitioning to intravenous epoprostenol or intravenous iloprost [60], suggesting the possibility that the total dose delivered and/or the short half-life (20 min) are limiting factors in achieving efficacy. Another application for inhaled iloprost has been in combination therapy for PAH. STEP II trial (iloprost inhalation solution Safety and pilot efficacy Trial in combination with bosentan for Evaluation in Pulmonary arterial hypertension) randomized 65 patients to add inhaled iloprost or placebo to bosentan [61]. Twelve weeks of combination therapy improved NYHA functional class, pulmonary hemodynamics, and time to clinical worsening compared to placebo. As a result of these trials, inhaled iloprost is approved by the FDA as monotherapy, or in combination with bosentan for PAH patients in functional class III or IV. The drug is administered via a dedicated aerosolization device at least six times a day. Mild symptoms of prostacyclin overdose such as headache, jaw pain, as well as cough have been reported. Although the current device is easy to use, has batteries and is light, the main drawback of inhaled iloprost is the frequency of administration (6–9 times a day), which makes compliance a major issue.
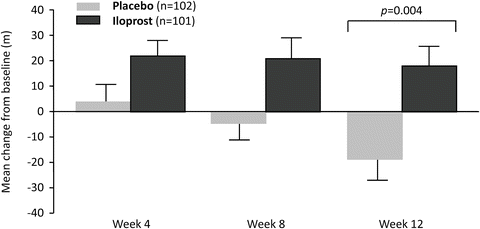

Fig. 13.3
Effect of inhaled iloprost and placebo on the mean (±SE) change from baseline in the distance walked in 6 min, according to an intention-to-treat analysis. The p value was obtained with Wilcoxon’s test for two independent samples. From [58]
The second inhaled prostacyclin available is inhaled treprostinil. In a cross-over study comparing acute effects of inhaled treprostinil with inhaled iloprost, both agents produced similar improvement in PVR [62]. However, the peak effect occurred later and the duration of effect lasted longer with inhaled treprostinil, suggesting the need for less frequent administration with inhaled treprostinil. Based on initial results as well as pilot studies in patients with PAH, a randomized, placebo-controlled study was conducted to evaluate the safety and efficacy of inhaled treprostinil in PAH patients on background oral therapies. TRIUMPH-I (Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension) randomized 235 patients who were stable on bosentan or sildenafil to receive placebo or inhaled treprostinil at a dose of nine inhalations four times a day. At 12 weeks, the placebo-corrected median increase in 6MWD was significantly greater in the treatment arm compared with placebo [63]. Although dyspnea score, NYHA functional class and time to clinical worsening were not significantly better in patients who received inhaled treprostinil, quality of life scores and plasma NT-pro-BNP levels were improved. Inhaled treprostinil is delivered four times a day via an OptiNeb ultrasonic nebulizer (NebuTec, Germany). Overall, inhaled treprostinil is well tolerated, with adverse effects consistent with prostacyclin effects, as well as cough.
Oral Prostacyclin Analogs
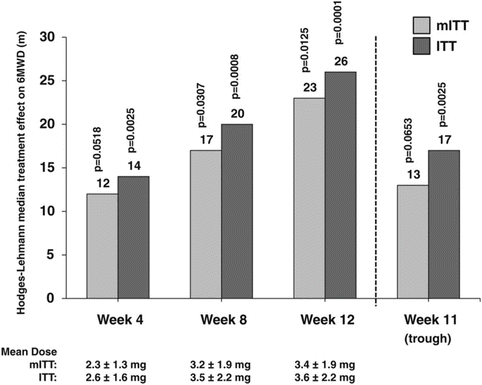
Given the cumbersome administration of parenteral prostacyclins and the limitations of inhaled therapies, oral prostacyclin analogs have been tested for their ease of administration. Beraprost is an oral prostacyclin analog available in Japan. A randomized trial conducted in Japan found improved survival with beraprost compared with conventional treatment (76 % versus 44 %, respectively) [64]. A 3-month randomized trial conducted in Europe showed significant improvements in 6MWD and functional class with beraprost, although hemodynamics did not improve [35]. A subsequent 12-month multicenter trial failed to show sustained benefit in the treatment arm, although there were significant improvements in 6MWD at 3 and 6 months [65]. For this reason, beraprost has not been approved in the US for the treatment of PAH. Most recently, the oral formulation of treprostinil was studied in three multicenter, randomized placebo-controlled trials. FREEDOM-C was a multicenter, double-blind, placebo-controlled trial that randomized 350 patients on background oral therapies (endothelin receptor antagonists and/or phosphodiesterase 5 inhibitors) [66]. Placebo-corrected median improvement in 6MWD was 11 m and not significantly greater than in controls (p = 0.07), although there were significant improvements in dyspnea score and in the combined 6MWD and dyspnea score. The increase from baseline in 6MWD was 4 m for patients who achieved a dose of <1 mg twice daily or discontinued due to side effects, 18 m for patients who achieved a week-16 dose of 1.25–3.25 mg twice daily and 34 m for patients who were on a dose of 3.5–16 mg twice daily, suggesting a dose-related efficacy. In the FREEDOM-C2 trial, 310 patients stable on endothelin receptor antagonists and/or phosphodiesterase 5 inhibitors were randomized to receive placebo or active drug over 16 weeks [67]. At the initiation of study drug, the first dose was lower than in the initial FREEDOM-C trial and the drug was better tolerated. The mean dose was 3.1 ± 1.9 mg twice a day. However, the placebo-corrected median difference in 6MWD did not achieve statistical significance (10 m, p = 0.089), nor did secondary endpoints. Lastly, FREEDOM-M was a multicenter, placebo-controlled double-blind study which evaluated oral treprostinil as monotherapy for PAH [68]. Three hundred and forty-nine patients were randomized over 12 weeks to receive active drug or placebo. There were significant improvements in the primary outcome, 6MWD, compared with placebo. In the intention-to-treat analysis, the improvement was 26 m (p = 0.0001) at peak and 17 m (p = 0.0025) at trough plasma study concentrations (Fig. 13.4). Secondary endpoints did not achieve statistical significance. The side effects most commonly encountered in the three studies were headache, diarrhea, nausea, flushing, and jaw pain. As a result of the FREEDOM studies in conjunction with recognition of the effectiveness of the previously approved non-oral formulations of treprostinil, oral treprostinil became the first oral prostacyclin analog approved by the FDA for the treatment of PAH. Oral treprostinil can be given twice or three times a day and should be administered with food to minimize gastrointestinal side effects. Three times daily dosing seems to be better tolerated than twice daily dosing, reflecting fewer swings in plasma levels. The dose is titrated as tolerated to achieve improvement in symptoms and exercise capacity. Caution needs to be exercised when patients cannot take oral medications, such as those undergoing surgical procedures, as long interruptions may necessitate its reintroduction at a lower dose and/or temporary use of parenteral prostacyclin replacement therapy.