Monocytes play a critical role in the pathophysiology of heart failure (HF), but few studies have evaluated the prognostic implications of an increased monocyte count in patients with HF and reduced ejection fraction (EF). The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) examined the effects of tolvaptan in patients with worsening HF and EF ≤40%. This post hoc analysis evaluated the primary end points of all-cause mortality and cardiovascular mortality or HF hospitalization in 3,717 patients. At baseline, 265 (7.1%) had an increased monocyte count defined by ≥800/μl. Patients with increased monocyte count tended to have an increased EF and were less likely to have a history of diabetes mellitus, hypercholesterolemia, or coronary revascularization but were more likely to have higher HF functional class and to be taking HF therapies such as diuretics, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and digoxin (p <0.05 for all comparisons). At median follow-up of 9.9 months, increased monocyte count was predictive of all-cause mortality (hazard ratio 1.27, 95% confidence interval 1.003 to 1.60, p = 0.047) but was not associated with cardiovascular mortality or HF hospitalization (hazard ratio 1.06, 95% confidence interval 0.87 to 1.30, p = 0.55). Similar results were seen when monocyte count was analyzed as a continuous variable. However, after adjustment for baseline clinical risk factors, monocyte count was not predictive of either primary end point. In conclusion, increased monocyte count occurs in a minority of patients hospitalized with HF and is associated with poor postdischarge prognosis. However, it does not contribute prognostic value above other more traditional risk factors.
Although the importance of monocytes and long-term inflammation in heart failure (HF) has been recognized, the prognostic significance of serum monocyte level is unclear. A study investigated the association between monocyte count and outcomes in patients with no previous HF immediately after acute myocardial infarction. These investigators found monocyte count to be an independent determinant of pump failure, HF rehospitalization, recurrent myocardial infarction, and cardiac death. One study has investigated the implications of serum monocyte count in patients with an established diagnosis of systolic HF. This small investigation of 30 patients with chronic HF and ejection fraction (EF) <40% found increased monocyte count to be predictive of cardiopulmonary adverse events, defined as worsening New York Heart Association (NYHA) functional class, hospitalization for cardiopulmonary complications, all-cause mortality (ACM), and cardiac transplantation. The Efficacy in Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) affords an opportunity to better characterize the implications of serum monocyte levels on clinical outcomes in a large cohort of patients hospitalized for worsening chronic HF and reduced EF.
Methods
The rationale and design of EVEREST have been previously reported. Briefly, EVEREST was a multicenter, randomized, double-blinded, placebo-controlled trial that examined the effects of tolvaptan, a vasopressin-2 receptor antagonist, in patients hospitalized for acute decompensated HF. Eligible patients were ≥18 years of age with EF ≤40%, signs of volume overload, NYHA class III or IV symptoms, and hospitalization for exacerbation of chronic HF. Criteria for exclusion included acute myocardial infarction at time of hospitalization, co-morbid conditions with an expected survival of <6 months, and refractory end-stage HF. Median follow-up was 9.9 months. Patients were randomized within 48 hours of hospitalization to receive oral tolvaptan (30 mg/day) or matching placebo in addition to standard HF therapy. The trial was conducted in full accordance with the Declaration of Helsinki and with institutional review board/ethics committee approval at all sites.
Discharged patients had prespecified follow-up visits at 1 week, 4 and 8 weeks, and every 8 weeks thereafter. The first outpatient visit occurred 7 days after discharge for those subjects discharged on or before day 10 after randomization or day 17 after randomization for those still in the hospital on day 10. Basic chemistries, body weight, vital signs, physical examination, and symptom scores were collected at randomization, hospital day 7 or discharge (whichever occurred first), and at each follow-up visit. Specific causes of death and reasons for rehospitalization were determined by an independent and blinded adjudication committee.
All laboratory testing was performed at 5 central facilities with consistency of results verified by cross validation among testing sites. The EVEREST protocol required a routine complete blood count with differential at the time of patient enrollment. Each complete blood count was performed using a commercially automated system. Serum monocyte count was not directly measured in EVEREST, and for this analysis it was calculated by multiplying total white blood cell (WBC) count by percent monocytes. Patients with missing data regarding baseline complete blood count or differential were excluded from analysis (n = 416). From the remaining 3,717 patients (90%) with validated baseline monocyte data, 2 groups were identified based on the presence or absence of monocytosis. Based on a review of the literature, monocytosis was defined as serum monocyte count ≥800/μl.
The present study used the 2 prespecified primary end points from the EVEREST outcome trial: ACM and a composite measurement of cardiovascular mortality (CVM) or HF hospitalization. Patient characteristics were compared between increased and normal monocyte count groups. All continuous variables were reported as mean ± SD if normally distributed or median (interquartile range) if non-normally distributed. Categorical variables were expressed as number (percentage). Baseline characteristics were compared using analysis of variance or Kruskal–Wallis and chi-square tests, as appropriate. Time to first event was analyzed using log-rank tests and Cox proportional hazard models. Kaplan–Meier curves were constructed for patients with increased and normal monocyte counts. The cube root of monocyte count was also evaluated as a continuous variable. Multivariable regression models were adjusted for multiple preselected baseline covariates that are known predictors of CVM and HF mortality and rehospitalization: tolvaptan assignment, age (years), gender, geographic region, EF (percentage), serum sodium (milliequivalents per liter), B-type natriuretic peptide (picograms per milliliter), serum urea nitrogen (milligrams per deciliter), systolic blood pressure (millimeters of mercury), QRS duration (milliseconds), NYHA functional class, intravenous inotrope requirement, total WBC count (per microliter), and use of β blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists. No suspicion for colinearity was present for estimates related to monocyte count in our final models (tolerance 0.89, variance inflation factor 1.12). Testing for interaction between HF cause (ischemic or nonischemic) and increased monocyte count was performed. Missing data were imputed using multiple imputation procedures. Effect sizes are reported as hazard ratio (HR) and 95% confidence interval (CI). Assumptions of proportional hazards and linearity of the continuous predictor were verified. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, North Carolina) at a conventional 2-tailed 5% significance level.
Results
Of the 3,717 patients with available monocyte data at baseline, 265 patients (7.1%) had an increased monocyte count ( Table 1 ). Patients with an increased monocyte count tended to be younger with higher EF, heart rate, diastolic blood pressure, and WBC count (p <0.03 for all comparisons). They were more likely to have NYHA class IV HF and to receive an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, diuretic, digoxin, and nitrate and were less likely to have a history of coronary artery bypass grafting, percutaneous coronary intervention, implantable cardioverter–defibrillator, diabetes mellitus, or hypercholesterolemia (p <0.05 for all comparisons). Table 2 lists complete baseline characteristics for all patients included in this analysis.
| Variable | Number | Mean ± SD | Median | 25th Percentile | 75th Percentile | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Monocyte count (cells/μl) | 3,717 | 477 ± 225 | 444 | 330 | 584 | 0 | 3,544 |
| White blood cell count (cells/μl) | 3,723 | 7,540 ± 2,670 | 7,160 | 5,910 | 8,700 | 1,910 | 59,070 |
| Monocytes (%) | 3,717 | 6.5 ± 2.6 | 6.2 | 4.9 | 8.0 | 0.0 | 22.9 |
| Characteristic | Monocyte Count/μl | p Value | |
|---|---|---|---|
| ≥800 (n = 265) | <800 (n = 3,452) | ||
| Tolvaptan | 148 (55.8%) | 1,707 (49.4%) | 0.045 |
| Age (years) | 64.1 ± 12.1 | 65.8 ± 11.8 | 0.028 |
| Men | 210 (79.2%) | 2,561 (74.2%) | 0.069 |
| Race | 0.098 | ||
| White | 234 (88.3%) | 2,965 (85.9%) | |
| Black | 10 (3.8%) | 248 (7.2%) | |
| Other | 21 (7.9%) | 239 (6.9%) | |
| Region of origin | <0.001 | ||
| Eastern Europe | 128 (48.3%) | 1,383 (40.1%) | |
| Western Europe | 18 (6.8%) | 465 (13.5%) | |
| North America | 48 (18.1%) | 1,031 (29.9%) | |
| South America | 71 (26.8%) | 573 (16.6%) | |
| Ischemic heart failure cause | 174 (66.4%) | 2,248 (66.0%) | 0.897 |
| Systolic blood pressure (mm Hg) | 122.0 ± 18.9 | 120.5 ± 19.8 | 0.222 |
| Diastolic blood pressure (mm Hg) | 75.3 ± 12.3 | 72.6 ± 12.7 | <0.001 |
| Heart rate (beats/min) | 83.9 ± 17.9 | 79.5 ± 15.4 | <0.001 |
| New York Heart Association class IV | 137 (51.7%) | 1,341 (38.9%) | 0.003 |
| Dyspnea | 243 (92.7%) | 3,092 (91.1%) | 0.364 |
| Jugular venous distention ≥10 cm | 73 (28.3%) | 922 (27.4%) | 0.743 |
| Rales | 216 (82.8%) | 2,768 (81.4%) | 0.596 |
| Peripheral edema ⁎ | 159 (60.0%) | 1,949 (56.5%) | 0.271 |
| Ejection fraction (%) | 29.2 ± 7.2 | 27.4 ± 8.0 | <0.001 |
| Serum urea nitrogen (mg/dl) | 29.2 ± 13.8 | 30.0 ± 16.3 | 0.370 |
| Creatinine (mg/dl) | 1.2 (1.0–1.6) | 1.3 (1.0–1.6) | 0.526 |
| Serum sodium (mEq/L) | 139.3 ± 5.6 | 139.7 ± 4.5 | 0.240 |
| Total white blood cell count | 10.1 ± 5.0 | 7.3 ± 2.3 | <0.001 |
| B-type natriuretic peptide (pg/ml) | 778.0 (311.0–1,717.2) | 688.0 (291.0–1,483.0) | 0.247 |
| Amino terminal pro–B-type natriuretic peptide (pg/ml) | 4,263.0 (2,450.0–13,874.0) | 4,674.2 (2,117.6–9,570.0) | 0.476 |
| QRS duration on electrocardiogram (ms) | 120.9 ± 33.9 | 127.5 ± 35.5 | 0.003 |
| Previous heart failure hospitalization | 212 (80.3%) | 2,724 (79.2%) | 0.666 |
| Previous coronary artery disease | 182 (68.7%) | 2,442 (70.8%) | 0.464 |
| Previous myocardial infarction | 135 (50.9%) | 1,758 (51.0%) | 0.993 |
| Hypertension | 190 (71.7%) | 2,439 (70.7%) | 0.719 |
| Hypercholesterolemia † | 97 (36.7%) | 1,691 (49.2%) | <0.001 |
| Diabetes mellitus | 78 (29.4%) | 1,358 (39.3%) | 0.001 |
| Previous coronary artery bypass grafting | 40 (15.1%) | 728 (21.1%) | 0.020 |
| Previous percutaneous coronary intervention | 24 (9.1%) | 626 (18.1%) | <0.001 |
| Chronic kidney disease | 62 (23.4%) | 911 (26.4%) | 0.284 |
| Peripheral vascular disease | 55 (20.8%) | 719 (20.9%) | 0.970 |
| Implantable cardioverter–defibrillator | 24 (9.1%) | 511 (14.8%) | 0.010 |
| Previous stroke | 38 (14.8%) | 387 (11.3%) | 0.090 |
| Chronic obstructive pulmonary disease | 27 (10.2%) | 340 (9.8%) | 0.858 |
| Baseline medication use | |||
| Diuretics | 263 (99.2%) | 3,356 (97.2%) | 0.047 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 237 (89.4%) | 2,906 (84.2%) | 0.023 |
| β-Blockers | 181 (68.3%) | 2,458 (71.1%) | 0.336 |
| Mineralocorticoid receptor antagonists | 154 (58.1%) | 1,882 (54.5%) | 0.257 |
| Digoxin | 167 (63.0%) | 1,632 (47.3%) | <0.001 |
| Nitrates | 126 (47.5%) | 1,308 (37.9%) | 0.002 |
| Intravenous inotropes | 10 (3.8%) | 149 (4.3%) | 0.674 |
| Outcomes | |||
| All-cause mortality | 76 (28.7%) | 896 (26.0%) | 0.331 |
| Cardiovascular mortality or heart failure hospitalization | 106 (40.0%) | 1,409 (40.8%) | 0.794 |
| Cardiovascular mortality | 58 (21.9%) | 687 (19.9%) | 0.437 |
| Cardiovascular mortality or cardiovascular hospitalization | 118 (44.5%) | 1,635 (47.4%) | 0.373 |
| Worsening heart failure ‡ | 90 (34.0%) | 1,249 (36.2%) | 0.468 |
| Heart failure hospitalization | 73 (27.5%) | 1,068 (30.9%) | 0.249 |
| Myocardial infarction hospitalization | 3 (1.1%) | 59 (1.7%) | 0.480 |
| Stroke hospitalization | 4 (1.5%) | 56 (1.6%) | 0.888 |
⁎ Peripheral edema was defined as slight/moderate/marked pedal or sacral edema.
† Patient-reported history of hypercholesterolemia.
‡ Worsening heart failure was defined as death from heart failure, hospitalization for heart failure, or unscheduled medical office visit for heart failure.
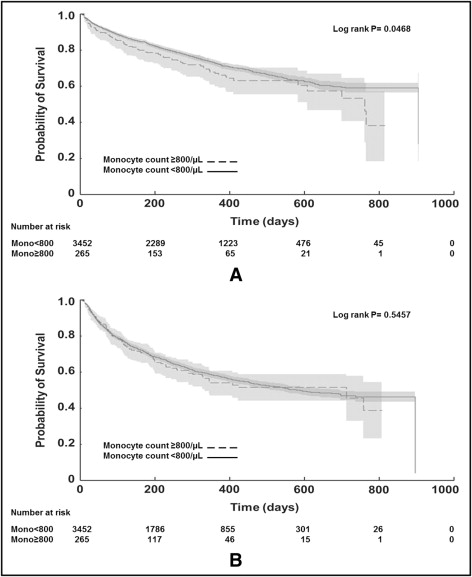
Patients with an increased monocyte count had higher ACM (29% vs 26%, HR 1.27, 95% CI 1.003 to 1.60, p = 0.047) but similar CVM/HF hospitalization (40% vs 41%, HR 1.06, 95% CI 0.87 to 1.30, p = 0.55; Figure 1 , Table 3 ). After adjusting for baseline risk factors, the predictive value of increased monocyte count on ACM was decreased to a trend (HR 1.24, 95% CI 0.96 to 1.59, p = 0.105). Increased monocyte count was not an independent predictor of CVM/HF hospitalization in the final multivariable analysis (HR 1.08, 95% CI 0.87 to 1.33, p = 0.479). The effect of increased monocyte count did not differ by HF cause (p >0.105; interaction analysis not shown).