Fig. 9.1
Relation between office and ambulatory systolic blood pressure in untreated hypertensive subjects enrolled in the “Progetto Ipertensione Umbria monitoraggio Ambulatoriale” (PIUMA) study. For any given value of office BP, the observed ambulatory BP varies considerably from the predicted value by linear regression equation. BP blood pressure
From a practical standpoint, the combined use of office and ambulatory BP identifies four different clinical categories of untreated subjects [6, 7]:
1.
Subjects who are normotensive by clinic BP and hypertensive by ambulatory BP (masked hypertension ; Fig. 9.2, upper left panel).
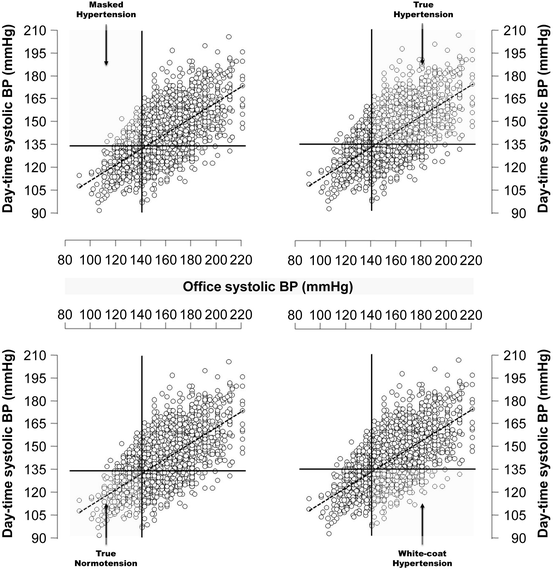

Fig. 9.2
Untreated hypertensive subjects enrolled in the “Progetto Ipertensione Umbria monitoraggio Ambulatoriale” (PIUMA) classified into 4 groups according to office (<140 or ≥140 mmHg) and day-time ambulatory (<135 or ≥135 mmHg) systolic blood pressure levels. BP blood pressure
4.
Subjects who are hypertensive based on office BP and normotensive by ambulatory BP (white coat hypertension, or isolated office hypertension; Fig. 9.2, lower right panel).
Notably, for both masked hypertension and white coat hypertension, the definition should be preferentially restricted to untreated patients. In treated patients, the office-ambulatory BP discrepancy might be conditioned by a different drop in one vs. the other pressure (because of the time of drug administration, the duration of the effect, and other reasons) and patients may have had originally a sustained rather than a white coat or masked hypertension condition.
Masked Hypertension
The phenomenon of masked hypertension (also referred to as “reverse white coat hypertension” or “white coat normotension”) is defined as a clinical condition in which a patient’s office BP level is normal (<140/90 mmHg), but ambulatory BP readings are in the hypertensive range (for instance; ambulatory daytime BP ≥135/85 mmHg).
This condition underlines the concept that hypertension may not be detected in these subjects on the grounds of traditional BP measurement. Reactivity to daily life stressors and some behavioral factors can selectively influence the phenomenon of masked hypertension. As depicted in a recent algorithm [6] proposed for the identification and management of subjects with masked hypertension (Fig. 9.3), several factors may be involved as potential determinants of masked hypertension [6]. They include pre-hypertension or high-normal BP, smoking status, regular alcohol consumption, male sex, diabetes, obesity, contraceptive use in women, sedentary habits, and exposure to high environmental stress [6].
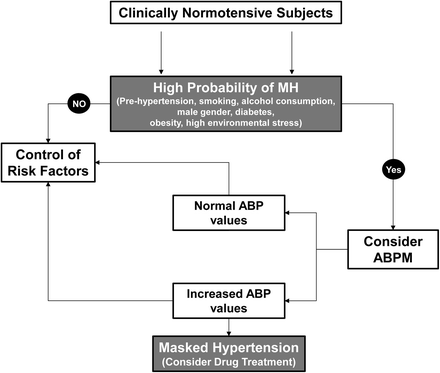

Fig. 9.3
Suggested algorithm for evaluating and treating masked hypertension. MH masked hypertension, ABP ambulatory blood pressure, ABPM ambulatory blood pressure monitoring
According to recent observations from cross-sectional studies, prevalence of masked hypertension ranges between 8 and 38 % [8]. Such variability has been attributed to different patient characteristics, populations studied, and different definitions of masked hypertension. Indeed, some studies were conducted in the general population, other in referred subjects with normotension, other in untreated or treated hypertensive patients, and other in elderly people, or specifically in men [6].
Individuals with masked hypertension present a higher prevalence of organ damage, particularly metabolic risk factors, left ventricular (LV) hypertrophy, increased carotid intima-media thickness, and impaired large artery distensibility when compared with subjects with a normal BP level both inside and outside the clinic or office [9].
Moreover, outcome studies have documented that masked hypertension increases cardiovascular risk, which appears to be comparable to that found in patients with concomitant in-office and out-of-office hypertension [6]. Available evidences show that subjects with masked hypertension have a 1.5–3-fold higher risk of major cardiovascular disease than those with normotension, and their risk is not different from that of patients with sustained hypertension [6, 8].
Some systematic overviews [6, 10, 11] evaluated the prognostic impact of masked hypertension. In this regard, the most recent meta-analysis [6] including 8 cohort studies (Table 9.1) reported quantitative data for cardiovascular prognosis. It showed that the risk of major cardiovascular disease was higher in subjects with masked hypertension than in the normotensive subjects regardless of the definition of masked hypertension based on self-measured BP (hazard ratio [HR]: 2.13; 95 % confidence interval [CI]: 1.35–3.35; p = 0.001) or 24-h ambulatory BP (HR: 2.00; 95 % CI: 1.54–2.60; p < 0.001) [6].
Table 9.1
Clinical studies that addressed the adverse prognostic impact of masked hypertension
Study | Selection criteria | MH detected by |
|---|---|---|
Bjorklund et al. [114] | 70-year-old men | ABPM |
Bobrie et al. [115] | Age ≥60 years | Home BP |
Fagard et al. [116] | Age ≥60 years | ABPM |
Hansen et al. [39] | Population sample aged 41–72 years | ABPM |
Ohkubo et al. [117] | Population sample aged ≥40 years | ABPM |
Mancia et al. [118] | Population sample aged 25–74 years | ABPM |
Mancia et al. [118] | Population sample aged 25–74 years | Home BP |
Pierdomenico et al. [17] | Age: 60 years (mean) | ABPM |
The high prevalence of masked hypertension remarks the priority of measuring out-of-office BP in a consistent proportion of people with apparently normal or well-controlled office BP. Nevertheless, the optimal strategy for detecting this condition is not yet clear and it is virtually impossible to screen for masked hypertension the totality of the general population [12]. Thus, it appears reasonable to restrict screening to those individuals at increased risk for cardiovascular complications (including patients with kidney disease or diabetes) and with potentially high pre-screen probability of masked hypertension (Fig. 9.3) [6].
White Coat Hypertension
White coat hypertension, also referred to as isolated office hypertension, is generally defined as a persistently elevated office BP (≥140/90 mmHg) with concomitant normal BP outside the office (<130–135/85 mmHg for daytime BP) [1].
Despite a large number of studies, it’s not easy to give an unequivocal definition of white coat hypertension based on results of 24-h ambulatory BP monitoring. While the usual definition of elevated office BP is out of discussion (≥140 mmHg for systolic and/or 90 mmHg for diastolic BP) [1–3], the upper reference limits of ambulatory BP used to define white coat hypertension differed across the available studies. The definition was based on both systolic and diastolic values in some studies and solely on diastolic values in others; some studies used the average ambulatory BP during the day and other used the average 24-h ambulatory BP [7, 8].
Such differences might seem clinically unimportant, but the prevalence of white coat hypertension and of the associated cardiac target-organ damage increased markedly when moving from more restrictive (lower) to more liberal (higher) limits of ambulatory BP normalcy over a relatively narrow range [14]. For example, the prevalence of LV hypertrophy, virtually absent below 120 mmHg and very low below 130 mmHg (6 %) for systolic BP, increases to 10.5 % when the limit was set to a more liberal value (140 mmHg) [14].
In addition, modest swings over a narrow range of presumably normal or nearly normal ambulatory BP may result in remarkable differences in the predicted cardiovascular risk [8, 15–18].
In some reports from our group [8, 15, 16], the subset with white coat hypertension was subdivided into two groups with low ambulatory BP values (average daytime ambulatory BP <130/80 mmHg) or values intermediate (between 130/80 and 131/86 mmHg in women or 136/87 mmHg in men). The differences in event-free survival between the normotensive group and the group with white coat hypertension defined restrictively were not significant, whereas the differences between the normotensive group and the white coat hypertension group defined more liberally were significant [8, 15, 16].
These data suggest that a daytime ambulatory BP <130 mmHg systolic and <80 mmHg diastolic may be defined “optimal” to identify individuals with white coat hypertension and low cardiovascular risk (i.e., risk not dissimilar from that of clinically normotensive subjects). To add further insight into the long-term clinical relevance of white coat hypertension, an International Collaborative Study examined individual data from 4 prospective cohort studies from the United States, Italy, and Japan [19]. In this study, 4,406 initially untreated subjects with essential hypertension and 1,549 healthy normotensive controls were followed for a median of 5.4 years [19]. Using the same restrictive ambulatory limits for the definition of white coat hypertension (average awake ambulatory BP <130/80 mmHg), the adjusted HR for stroke was 1.15 (95 % CI: 0.61–2.16) in the white coat hypertension group (p = 0.66) and 2.01 (95 % CI: 1.31–3.08) in the ambulatory hypertension group (p = 0.001) compared with the normotensive group. However, the incidence of stroke tended to increase in the white coat hypertension group in the long run, and the corresponding hazard curve crossed that of the ambulatory hypertension group by the ninth year of follow-up [19]. These data raised the hypothesis, to be tested in future studies, that white coat hypertension might not be a fully benign condition on the long-term, being a sort of intermediate condition between normotension and established hypertension [20–25].
Although controversy still exists regarding the prognostic impact of this condition, current evidence suggests a treatment based on lifestyle measures in the low-risk stratum of subjects with white coat hypertension under the conditions of restricted definition (daytime ambulatory BP <130/80 mmHg), absence of important comorbid conditions and target organ damage, and adequate follow-up [20–25].
Surrogate Outcome Markers
Surrogate outcome markers may provide a significant contribution to early diagnosis and outcome prediction in hypertension. They are useful noninvasive tools for designing and evaluating therapeutic programs and are increasingly employed as predictive endpoints for treatment [26].
In this context, the association between office BP and hypertensive target organ damage (TOD) usually is relatively poor. Conversely, the closer correlation between TOD and ambulatory BP is well-established regardless of whether the damage is quantified in the heart (LV hypertrophy or dysfunction), in the kidney (microalbuminuria or overt proteinuria), in the brain (cerebral lacunae or white matter lesions as identified by nuclear magnetic resonance), or in the small and large arteries [27].
Left Ventricular Hypertrophy
In the earlier studies by Drayer et al. [28] and Devereux et al. [29], ambulatory BP correlated more closely with LV mass than did office BP. Subsequently, several studies confirmed these results and actually there is a general consensus regarding the closer correlation between LV mass and ambulatory over clinic BP (Fig. 9.4) [30–32].
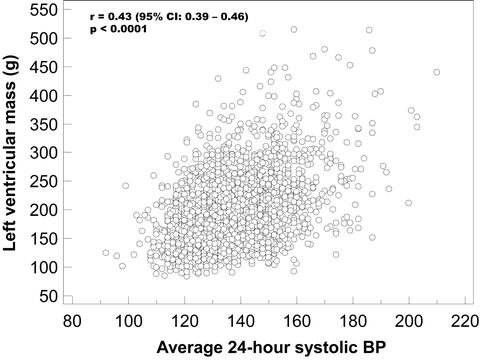

Fig. 9.4
Association between average 24-h systolic blood pressure and left ventricular mass in untreated hypertensive subjects enrolled in the “Progetto Ipertensione Umbria Monitoraggio Ambulatoriale” (PIUMA) study. BP blood pressure
More recent data have also provided evidence that serial changes in LV mass show a closer association with the changes in ambulatory BP than with those in clinic BP [33].
In this context, the Study on Ambulatory Monitoring of Pressure and Lisinopril Administration (SAMPLE) evaluated whether in hypertensive patients with a marked echocardiographic LV hypertrophy regression of hypertrophy by a 12-month treatment was more closely related to reduction in 24-h average than in office BP [34]. Treatment consistently reduced office BP, 24-h average BP, and LV mass index. The reductions in office and 24-h BP showed a limited relationship to each other, while only the latter, but not the former, showed a significant relationship with the degree of the LV hypertrophy regression [34].
More recently, a report from the Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study [33] demonstrated that the changes in LV mass during BP-lowering treatment was significantly associated with the changes in 24-h systolic BP (r = 0.40), diastolic BP (r = 0.33), and pulse pressure (r = 0.35). Weaker associations were found with the changes in clinic BP (r = 0.32, 0.31 and 0.16, respectively). When 24-h systolic BP and 24-h diastolic BP were forced into the same model, only 24-h systolic BP achieved significance. Similarly, when 24-h systolic BP and 24-h pulse pressure were included into the same model as covariates, only 24-h systolic BP was associated to statistical significance [33].
Other Organ Damages
A large number of studies have investigated whether, on a cross-sectional basis, other organ damages accompanying hypertension are more closely related to ambulatory than to office BP. Microalbuminuria and albumin excretion rate are better predicted by ambulatory BP than by office BP [35].
The European Lacidipine Study on Atherosclerosis (ELSA) [34] demonstrated that either the number of plaques or the size of the thickening was more closely related to 24-h average systolic BP and ambulatory pulse pressure than to the corresponding office values. Indeed, 24-h average systolic BP or pulse pressure values were only second to age in their correlation with carotid artery wall status, their importance being also greater than that seen for serum cholesterol and other components of the lipid profile [34].
Similarly, Asmar et al. found a closer association of arterial distensibility by carotid-femoral pulse velocity with ambulatory BP than with office BP [36].
Taken together, these data provide substantial evidence that the adverse effects of hypertension are related to the average ambulatory BP level to which target organs have been exposed over time and that the more accurate prognostic value of ambulatory over clinic BP is partially due to a more strict association with hypertension-related organ damage.
Ambulatory Blood Pressure Components
Historically, average 24-h, daytime (awake), and night-time (asleep) BP values have been the principal components of the ambulatory BP profile to be investigated as prognostic markers [5, 37]. However, new statistical techniques and the ability to handle large volumes of data with computer-assisted analysis have led to studies that described different aspects of ambulatory readings and several information provided by ambulatory BP monitoring [2, 3].
A new language emerged, with such terms as “nocturnal BP dipping,” “morning surge in BP,” “ambulatory pulse pressure,” “ambulatory arterial stiffness index (AASI) ,” “average heart rate and heart rate period,” “BP variability and variability-ratios,” and “BP load” [1, 5, 37]. Health professionals have thus to adjust and incorporate this new knowledge into their clinical practice.
Average Blood Pressure
One of the first studies that addressed the prognostic value of ambulatory BP in a general population sample was the Ohasama study [38]. In this Japanese population (1542 subjects, 565 men and 977 women, aged ≥40 years), the association between BP levels and mortality was more distinctive for the 24-h ambulatory BP than it was for the office BP. Specifically, when both 24-h and office BP values were included in a multivariable Cox model, only the systolic ambulatory BP was related significantly to the increased risk of cardiovascular mortality [38].
Similar results were also observed in a Danish general population cohort [39]. A recent meta-analysis of 7,030 subjects [40] showed that average day-time ambulatory BP was superior to office BP in predicting cardiovascular events. In multivariate-adjusted continuous analyses, both office and ambulatory BP predicted cardiovascular outcome. However, in fully adjusted models, including both office and ambulatory BP, office BP lost its predictive value, whereas systolic day-time ambulatory BP retained their prognostic significance.
More recently, measurement of night-time BP proved to yield additional prognostic data in terms of all-cause mortality and cardiovascular events. In the Dublin Outcome Study [41], a large observational registry of subjects who underwent ambulatory BP before treatment, after correction for several confounders, ambulatory BP was superior to office BP for prediction of cardiovascular mortality and night-time ambulatory BP was the most potent ambulatory BP component for prediction of outcome [41].
A cohort study of 7458 patients in six countries from Europe, Asia, and South America [42] found that both day-time and night-time BP predicted all cardiovascular events. Nevertheless, night-time BP, adjusted for day-time BP, independently predicted total, cardiovascular, and non-cardiovascular mortality [42].
In elderly subjects with isolated systolic hypertension [43], ambulatory systolic BP was a significantly better predictor of cardiovascular and cerebrovascular events than conventional BP measurement. During follow-up, 98 patients developed a major cardiovascular event and after adjustment for age, sex, office BP, active treatment, previous events, cigarette smoking, and residence in the western Europe, the average night-time systolic BP was a significant predictor of total, cardiac, and cerebrovascular future events, whereas the average daytime BP did not yield statistical significance [43]. In this study, for every 10 mmHg increase in night-time systolic BP, the hazard rate for cardiovascular events was 1.20 (95 % CI: 1.08–1.35), while those for cardiac and cerebrovascular events were 1.16 (95 % CI: 1.02–1.33) and 1.31 (95 % CI: 1.06–1.62), respectively [43].
Notably, the interpretation of these averages in clinical practice is obviously dependent on the definition of normalcy. Although reference values for average ambulatory BP are still uncertain for the paucity of data allowing a shared definition dividing up normotension from hypertension, currently accepted limits of normalcy for average ambulatory BP based on several population-based data banks and longitudinal studies in hypertension are reported in Table 9.2 [1].
Table 9.2
Recommended limits of normalcy for ambulatory blood pressure
Limits of normalcy | Value |
|---|---|
Average 24-h | <130/80 mmHg |
Average day-time | <135/85 mmHg (optimal: <130/80 mmHg) |
Average night-time | <120/70 mmHg |
Day–Night Blood Pressure Changes
Intra-arterial studies with beat-to-beat recording in ambulant subjects showed that, in normotensive individuals, BP is characterized by a circadian pattern, with values tending to peak during the day-time hours and then falling to a nadir at night [44, 45]. In this context, 24-h ambulatory BP monitoring appears to be a valid tool to investigate the diurnal BP changes associated with the sleep–wake cycle, because it has been demonstrated that intra-arterial 24-h BP profile is similar in the absence and in the presence of concomitant noninvasive BP monitoring [46].
Several studies addressed the issue of the prognostic value of night-time BP and day–night BP changes, particularly in individuals whose nocturnal BP remains elevated [47]. Typically, “non-dippers” are defined by a reduction in BP by less than a given percentage from day to night, and the subjects out of this definition are classified as “dippers.” The threshold values for classification ranged from 10 % to 10/5 mmHg, up to 0 % (i.e., no reduction in BP from day to night or a higher BP during night than during day) [48].
Several reports from independent centers showed that prevalence of LV hypertrophy [49], cerebrovascular disease [50, 51], and microalbuminuria [52] was higher among subjects with blunted or abolished fall in BP from day to night than individuals with normal day–night BP difference.
Furthermore, day–night BP changes significantly refined cardiovascular risk stratification above office BP and other traditional risk markers [53, 54].
Yamamoto et al. [55] demonstrated that the degree of ambulatory BP reduction from day to night at the baseline assessment was significantly (p < 0.01) smaller in the group with subsequent cerebrovascular events than in the group with no future events. In older patients with isolated systolic hypertension, the Syst-Eur study found that cardiovascular risk increased with a higher night:day ratio of systolic BP (i.e., in patients more likely to be non-dippers) independent of the average 24-h BP [43]. Similarly, Ohkubo et al [56] showed an increased cardiovascular mortality in “non-dippers” (relative risk [RR]: 2.56, p = 0.02) and “reverse-dippers” (RR: 3.69, p = 0.004) in comparison with “dippers.”
Nevertheless, it is important to mention that night-time BP may lose its prognostic significance in hypertensive subjects with significant sleep disturbances during overnight monitoring, which may occur for the compressive, tactile, and sonorous stimuli produced by repeated cuff inflations [57].
Recent findings from our group supported this hypothesis, suggesting that the prognostic impact of day–night BP changes should be investigated including the perceived quantity of sleep as effect modifier in outcome analyses [57]. Specifically, we followed 2934 initially untreated hypertensive subjects and we assessed the perceived quantity of sleep during overnight BP monitoring [57]. Overall, 58.7, 27.7, 9.7, and 4.0 % of subjects reported a sleep duration perceived as usual (group A), <2 h less than usual (group B), 2–4 h less than usual (group C), and >4 h less than usual (group D). Daytime BP did not differ across the groups (all p not significant). Night-time BP increased from group A to D (124/75, 126/76, 128/77, and 129/79 mmHg, respectively; all p for trend <0.01). Over a median follow-up period of 7 years, there were 356 major cardiovascular events and 176 all-cause deaths. Incidence of total cardiovascular events and deaths was higher in the subjects with a night/day ratio in systolic BP >10 % compared with those with a greater day–night BP drop in the group with perceived sleep duration as usual or <2 h less than usual (both p < 0.01), not in the group with duration of sleep ≥2 h less than usual (all p not significant). Notably, in a Cox model, the independent prognostic value of night-time BP for total cardiovascular end points and all-cause mortality disappeared in the subjects with perceived sleep deprivation ≥2 h.
Blood Pressure Variability
BP is characterized by marked fluctuations occurring within a 24-h period. Rather than representing “background noise,” or a phenomenon occurring at random, these variations are thought to be the result of complex interactions between extrinsic environmental and behavioral factors and intrinsic cardiovascular regulatory mechanisms [58].
Although the adverse cardiovascular consequences of hypertension are thought to depend largely on mean BP values, the hypothesis that increased short-term BP variability may contribute to a worse prognosis in hypertensive patients received a great deal of attention.
Ambulatory BP variability is generally estimated by computing the standard deviation (SD) of the mean systolic and diastolic BP values over a 24-h period or, more appropriately, day-time and night-time periods separately in order to exclude the day–night BP dip from the estimate of this kind of variability. However, other estimates, including weighted standard deviation and coefficient of variation, have been proposed in the last years [59–61].
Importantly, prospective studies have provided evidence that an increase in BP variability within 24-h independently predicts progression of subclinical organ damage, cardiovascular events, and cardiovascular mortality [62–64]. Overall, these evidences support the concept that the adverse cardiovascular consequences of high BP depend on BP variability as well as on mean BP [62–64].
Of particular interest are the findings of a 2007 analysis of the PIUMA study [64]. In this study, BP variability was estimated by the SD of daytime or night-time systolic and diastolic BP. A BP variability ≤ or > the group median (12.7/10.4 mmHg for day-time systolic and diastolic BP and 10.8/8.9 mmHg for night-time systolic and diastolic BP) identified subjects at low or high BP variability. During follow-up, there were 167 new cardiac and 122 new cerebrovascular events. The rate of cardiac events (×100 person–years) was higher (all p < 0.05) in the subjects with high than in those with low BP variability (day-time systolic BP: 1.45 vs. 0.72, day-time diastolic BP: 1.29 vs. 0.91; night-time systolic BP: 1.58 vs. 0.62; nigh-time diastolic BP: 1.32 vs. 0.85). The rate of cerebrovascular events was also higher (all p < 0.05) in the subjects with high than in those with low BP variability [64]. In a multivariate analysis, after adjustment for several confounders, a high night-time systolic BP variability was associated with a 51 % (p = 0.024) excess risk of cardiac events. The relation of day-time BP variability to cardiac events and that of day-time and night-time BP variability to cerebrovascular events lost significance in the multivariate analysis [64].
More recently, these findings have been examined in a large multinational cooperative database including 7,112 untreated hypertensive patients enrolled in 6 prospective studies [65]. In a multivariable Cox model, night-time BP variability (as estimated by SD) was an independent predictor of all-cause mortality, cardiovascular mortality, and cardiovascular events. In contrast, day-time BP variability was not an independent predictor of outcomes in any model. In fully adjusted models, a night-time systolic BP SD ≥ 12.2 mmHg was associated with a 41 % greater risk of cardiovascular events, a 55 % greater risk of cardiovascular death, and a 59 % increased risk of all-cause mortality compared with an SD < 12.2 mmHg. The corresponding values for a diastolic BP SD of ≥7.9 mmHg were 48 %, 132 %, and 77 %, respectively. The addition of night-time BP variability to fully adjusted models had a significant impact on risk reclassification and integrated discrimination for all outcomes (relative integrated discrimination improvement for systolic BP variability: 9 % cardiovascular events, 14.5 % all-cause death, 8.5 % cardiovascular death; for diastolic BP variability: 10 % cardiovascular events, 19.1 % all-cause death, 23 % cardiovascular death, all P < 0.01).
Pulse Pressure
Age-related increases in BP are mainly attributable to an increase in systolic BP with a parallel steadiness, or slight decrease in diastolic BP [66]. This leads to a widening in pulse pressure. Stiffening of large arteries and decreased arterial compliance are recognized key features of aging, which largely account for the changes in pulse pressure occurring from 50 years of age onwards [66].
Because pulse pressure is strongly affected by the alerting reaction evoked by the clinical visit [67], it has been suggested that 24-h ambulatory pulse pressure may better reflect “true” pulse pressure during normal daily life.
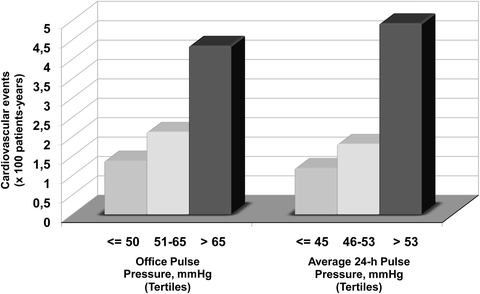
A significant association has been noted in several studies between pulse pressure and subsequent rate of cardiovascular morbid events, and such association was in part independent from the effects of systolic and diastolic BP [68–75]. In this context, some analyses of the PIUMA study elucidated the independent prognostic value of ambulatory pulse pressure [73, 74]. The first report included 2,010 initially untreated and uncomplicated subjects with essential hypertension (mean age, 51.7 years; 52 % men) [74]. All subjects underwent baseline procedures including 24-h noninvasive ambulatory BP monitoring and the mean duration of follow-up was 3.8 years (range, 0–11 years). In the three tertiles of the distribution of average 24-h pulse pressure (Fig. 9.5, right panel), the rate of total cardiovascular events was 1.19, 1.81, and 4.92, respectively (log-rank test, p < 0.01). Similar results were obtained for office pulse pressure (Fig. 9.5, left panel). However, after controlling for several independent risk markers including white coat hypertension and non-dipper status, we found that ambulatory pulse pressure was associated with the biggest reduction in the −2 log likelihood statistics for cardiovascular morbidity (p < 0.05 versus office pulse pressure). For any given level of office pulse pressure, cardiovascular morbidity and mortality markedly increased with average 24-h ambulatory pulse pressure.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


