Coronary artery calcium score (CACS) is a strong predictor of adverse cardiovascular events in the general population. Recent data confirm the prognostic utility of single-photon emission computed tomographic (SPECT) imaging in end-stage renal disease, but whether performing CACS as part of hybrid imaging improves risk prediction in this population is unclear. Consecutive patients (n = 284) were identified after referral to a university hospital for cardiovascular risk stratification in assessment for renal transplantation. Participants underwent technetium-99m SPECT imaging after exercise or standard adenosine stress in those unable to achieve 85% maximal heart rate; multislice CACS was also performed (Siemens Symbia T16, Siemens, Erlangen, Germany). Subjects with known coronary artery disease (n = 88) and those who underwent early revascularization (n = 2) were excluded. The primary outcome was a composite of death or first myocardial infarction. An abnormal SPECT perfusion result was seen in 22% (43 of 194) of subjects, whereas 45% (87 of 194) had at least moderate CACS (>100 U). The frequency of abnormal perfusion (summed stress score ≥4) increased with increasing CACS severity (p = 0.049). There were a total of 15 events (8 deaths, and 7 myocardial infarctions) after a median duration of 18 months (maximum follow-up 3.4 years). Univariate analysis showed diabetes mellitus (Hazard ratio [HR] 3.30, 95% CI 1.14 to 9.54; p = 0.028), abnormal perfusion on SPECT (HR 5.32, 95% CI 1.84 to 15.35; p = 0.002), and moderate-to-severe CACS (HR 3.55, 95% CI 1.11 to 11.35; p = 0.032) were all associated with the primary outcome. In a multivariate model, abnormal perfusion on SPECT (HR 4.18, 95% CI 1.43 to 12.27; p = 0.009), but not moderate-to-severe CACS (HR 2.50, 95% CI 0.76 to 8.20; p = 0.130), independently predicted all-cause death or myocardial infarction. The prognostic value of CACS was not incremental to clinical and SPECT perfusion data (global chi-square change = 2.52, p = 0.112). In conclusion, a perfusion defect on SPECT is an independent predictor of adverse outcome in potential renal transplant candidates regardless of the CACS. The use of CACS as an adjunct to SPECT perfusion data does not provide incremental prognostic utility for the prediction of mortality and nonfatal myocardial infarction in end-stage renal disease.
Renal transplantation remains the most successful and cost-effective treatment for patients with end-stage renal disease (ESRD), significantly improving cardiovascular (CV) outcomes compared with maintenance dialysis. Even after transplantation, however, patients remain at high risk of long-term CV complications. To ensure that graft survival is not limited by premature CV death, both US and UK regulatory bodies recommend noninvasive CV assessment of those transplant candidates with multiple risk factors or diabetes, although there is no clear guidance on which imaging method to use. The current suggestion is to adopt an imaging protocol for CV risk stratification according to “best local expertise.” Accordingly, many transplant centers continue to use stress myocardial perfusion scintigraphy because of longstanding data supporting its prognostic utility in ESRD. Despite this, myocardial perfusion scintigraphy has poor positive predictive value for identifying coronary artery stenosis on invasive angiography. Moreover, it is not able to detect subclinical atherosclerosis, potentially predisposing the patient in the longer term to subsequent obstructive CV events. Hybrid single-photon emission computed tomographic (SPECT)/CT imaging offers an attractive opportunity to combine anatomic measures of coronary artery calcification alongside a functional assessment of myocardial ischemia. Coronary artery calcium score (CACS) is a surrogate marker of atherosclerotic burden and a strong predictor of adverse CV events in subjects at intermediate risk from the general population. The predictive role of CACS in subjects with ESRD, however, is less certain. Despite the very high burden of coronary calcification in this population, there is only a modest association between CACS and perfusion defects. In the present study, we hypothesize that CACS will provide an incremental benefit for the prediction of death and first myocardial infarction (MI) in patients with ESRD beyond that provided by perfusion defect scores on myocardial perfusion imaging.
Methods
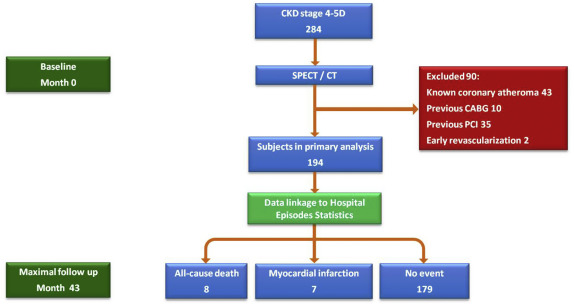
Consecutive patients with chronic kidney disease (CKD) stage 4 to 5D were identified after referral to Queen Elizabeth Hospital Birmingham for CV risk stratification as part of a pretransplant screening work-up from January 2011 to December 2013. In accordance with current guidelines compiled by a Joint Working Party of The British Transplantation Society and The Renal Association, subjects were referred for noninvasive CV risk assessment if they fulfilled any of the following criteria: age ≥50years, diabetes, suspected angina, or known ischemic heart disease. Those subjects with a history of MI, coronary atheroma or stenosis on angiography, or previous percutaneous or surgical revascularization were excluded from the present study ( Figure 1 ). Formal ethical approval was not required because this study was a retrospective assessment of solely clinical data and was therefore regarded as a health outcomes evaluation. The conduct and reporting of this study was guided by the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Demographic and anthropometric data were collected on all patients through review of patient electronic records. In addition, a standard prescan assessment involving a detailed patient interview was performed to obtain information on symptoms, CV risk factors, previous CV events, and medication. A Duke pretest probability of coronary artery disease (CAD) was calculated at the time of the imaging study. Routine hematology and biochemistry at the time of the test were also recorded. Diabetes mellitus (DM) was defined as a fasting glucose >126 mg/dl, history of DM, diabetic nephropathy, or currently receiving hypoglycemic treatment. Hypertension was defined as an office blood pressure >140/90 mm Hg or currently taking antihypertensive medication. Hypercholesterolemia was defined as a serum cholesterol of >193 mg/dl or currently taking lipid reduction therapy. A history of CV disease was defined as having any of the following: CAD (MI, previous percutaneous, or surgical revascularization), heart failure, stroke, and peripheral vascular disease. Significant family history of CV disease was defined as a first degree relative with a history of MI or ischemic stroke in men younger than 55 years and in women younger than 65 years.
Patients were asked to discontinue β blockers, rate-limiting calcium channel blockers, and caffeine products 24 hours before testing, and nitrate compounds were discontinued >6 hours before testing. All participants underwent 2-day stress-rest technetium-99m SPECT imaging with exercise treadmill or standard adenosine stress (140 μg/kg/min for 6 minutes) in those unable to achieve 85% maximal heart rate; and multislice CACS was performed as routine. CT-based attenuation correction was performed in all patients during reconstruction of the SPECT data (Symbia T16, Siemens, Erlangen, Germany).
SPECT myocardial perfusion images were visually analyzed by 2 experienced observers (RPS and BH) blinded to outcome variables (Quantitative Perfusion SPECT; Hermes Medical Solutions, Stockholm, Sweden). In addition to examination of raw images in cine mode, both nonattenuated and attenuated images were reviewed, and a report produced consistent with recommendations outlined in the American Society of Nuclear Cardiology Imaging Guidelines for Nuclear Cardiology Procedures. Short-axis and vertical long-axis tomograms were divided into 17 segments for each study, and segmental tracer uptake was evaluated using a validated semiquantitative 5-point scoring system (0, normal; 1, equivocal; 2, moderate; 3, severe reduction of radioisotope uptake; and 4, absence of detectable tracer uptake). The summed stress and rest scores were obtained by adding the scores of the 17 segments of the respective images. The sum of the differences between each of the 17 segments from these images was defined as the summed difference score, representing the amount of ischemia. These indexes were converted to the percentage of total myocardium involved with stress, ischemic, or fixed defects by dividing the summed scores by 68 (the maximum potential score = 4 × 17) and multiplying by 100. The presence of abnormal perfusion was defined as a summed stress score of 4 or greater. A stress-induced total perfusion defect size (PDS) >15% or an ischemic PDS >10% defined high risk for cardiac events. Cardiac volumes and left ventricular (LV) ejection fraction were also calculated from the gated SPECT images.
The CACS was calculated according to Agatston et al by the same 2 independent observers blinded again to outcome data. Lesions were manually traced on CT images before semiautomatic quantification-derived vessel-specific scores were summated to yield the total CACS (syngo.via; Leonardo; Siemens Medical Solutions, Forchheim, Germany). Minimal, mild, moderate, and severe coronary calcification were defined as Agatston scores of 0 to 10 U, 11 to 100 U, 101 to 400 U, and >400 U, respectively.
The primary outcome was a composite of all-cause death or MI. Myocardial infarction was defined as a clinical (or pathologic) event caused by myocardial ischemia where there is evidence of myocardial injury or necrosis as defined by an increase and/or decrease of cardiac biomarkers in the presence of typical symptoms or electrocardiographic changes, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. Patients who had revascularization within 90 days of the imaging study were identified and excluded from the analysis to avoid inclusion of outcomes that may have been driven temporally by the SPECT/CT result. The event of all-cause death was examined separately as a secondary outcome. Patients who underwent renal transplant surgery during the study period were also identified.
Patient follow-up data were retrieved by an observer blinded to the clinical and imaging data (WEM). Every patient in the National Health Service has a unique identifier which enables outcomes to be tracked using the Hospital Episodes Statistics (HES) database, an administrative data warehouse containing admissions to all National Health Service hospitals in England. It contains detailed records relating to individual patient treatments, with data extraction facilitated using codes on procedural classifications ( Office of Population Censuses and Surveys Classification of Interventions and Procedures, Fourth revision ) and medical classifications ( World Health Organization International Classification of Disease, Tenth revision ). With regard to outcome analysis, HES data alone have the limitation of only capturing deaths occurring in a hospital setting. To obtain the complete mortality list, the study cohort was also cross-referenced with mortality data from the Office for National Statistics, which collects information on all registered deaths in the UK. All outcomes were further verified by cross-referencing with individual hospital case notes held electronically.
Statistical analyses were performed with Stata, version 12 (StataCorp LP, College Station, Texas) and SAS (Statistical Analysis System, SAS Institute Inc., Cary, North Carolina). Data are expressed as mean ± SD, median (interquartile range), or frequency (%), unless otherwise stated. The normality of distribution for continuous variables was determined using normality plots and the Kolmogorov–Smirnov test. Baseline characteristics of the population were examined by CACS category and SPECT results. The Kruskal–Wallis analysis of variance was used to identify significant differences in central tendencies of continuously scaled variables between groups. Contingency table analysis was performed using the chi-square or Fisher’s exact tests where appropriate.
Annualized event rates are expressed as the number of patients having first MI or all-cause death as a proportion of the number of patients at risk divided by the number of patient-years follow-up. Kaplan–Meier analysis of outcomes were based on discrete CACS categories (0 to 10, 11 to 100, 101 to 400, and >400 U) and SPECT categories (normal, total LV PDS dichotomized at 15%, ischemic PDS dichotomized at 10%). The date of the imaging test was used as time zero. In view of the beneficial CV effects of renal transplantation, those patients undergoing renal transplantation were censored at the time of the procedure. Two-sided log-rank tests were used to determine significance. Univariate and multivariate Cox proportional hazards models were used to identify the association between time-to-event and baseline clinical characteristics, SPECT and CACS results. Multivariate Cox regression analyses were also repeated using follow-up data not censored for transplantation. The change in the global chi-square statistic was calculated to determine the incremental prognostic value of clinical, SPECT, and CACS data. A p value <0.05 was considered statistically significant for all analyses.
Results
In total, 284 consecutive patients (CKD stage 4 to 5D) with imaging performed from March 2011 to December 2013 were identified; of those, 88 had CAD at baseline. A further 2 subjects without a previous diagnosis of coronary atheroma underwent early revascularization (1 coronary artery bypass graft surgery and 1 percutaneous coronary intervention) after SPECT demonstrated a reversible PDS ≥10%, leaving 194 subjects available for inclusion in the present analysis ( Figure 1 ).
The baseline characteristics of the study cohort are summarized in Table 1 . Mean age was 56 years, 60% were men, 33% were diabetic, and 82% were hypertensive. Most patients were asymptomatic (75%). Two-thirds of patients had at least mild CACS (65%), and over a quarter had severe CACS (27%). In those with an abnormal SPECT result (28%), almost half (42%) had a total PDS ≥15% and a third (30%) had an ischemic PDS ≥10%.
| Variable | n = 194 |
|---|---|
| Age (years) | 56.3 ± 10.2 |
| Male | 117 (60%) |
| White | 128 (66%) |
| Asian | 49 (25%) |
| Afro-Caribbean | 12 (6%) |
| Other ethnicity | 4 (2%) |
| Body mass index (kg / m 2 ) | 27.5 ± 5.0 |
| Diabetes mellitus | 64 (33%) |
| Hypertension ∗ | 159 (82%) |
| Hypercholesterolemia † | 133 (69%) |
| Current smoker | 36 (19%) |
| Family history of coronary artery disease | 38 (20%) |
| Number of cardiac risk factors | 2.3 ± 1.0 |
| Duke pre-test probability (%) | 5 (3 – 8) |
| Symptomatic chest pain | 48 (25%) |
| Typical angina / atypical / non-cardiac | 10 (5%) / 20 (10%) / 18 (9%) |
| Hemoglobin (g/ L) | 111 ± 16 |
| Total cholesterol (mg / dL) | 185 ± 46 |
| Calcium (mg / dL) | 9.00 ± 0.64 |
| Phosphate (mg / dL) | 4.30 ± 1.24 |
| Parathyroid hormone, (median pg / mL [IQR]) | 21.8 (13.1 – 39.9) |
| Uric acid (mg / dL) | 7.13 ± 1.98 |
| CACS (median Agatston units [IQR]) | 52 (0 – 509) |
| CACS severity | |
| 0 – 10 | 68 (35%) |
| 11 – 100 | 39 (20%) |
| 101 – 400 | 35 (18%) |
| >400 | 52 (27%) |
| Ability to perform exercise stress | 112 (58%) |
| METS achieved ‡ | 6.7 ± 3.4 |
| Stress electrocardiogram result Normal / Equivocal / Abnormal | 130 (67%) / 39 (20%) / 25 (13%) |
| Left ventricular ejection fraction (median % [IQR]) | 56 (50 – 62) |
| Abnormal SPECT § | 43 (22%) |
| Total perfusion deficit score (% LV) | 3.9 ± 8.9 |
| Ischemic perfusion deficit score (% LV) | 1.6 ± 3.8 |
| Total perfusion deficit score ≥ 15% | 18 (9%) |
| Ischemic perfusion deficit score ≥ 10% | 13 (7%) |
| Medications | |
| Aspirin | 71 (37%) |
| Thienopyridine | 9 (5%) |
| Beta-blocker | 79 (41%) |
| ACE inhibitor / angiotensin receptor blocker | 86 (44%) |
| Calcium channel blocker | 97 (50%) |
| Loop diuretic | 66 (33%) |
| Statin | 123 (63%) |
| Insulin | 42 (22%) |
∗ Defined as an office blood pressure of >140/90 mm Hg or currently taking antihypertensive medications.
† Defined as a fasting serum cholesterol of >193 mg/dl or currently taking lipid reduction therapy.
‡ In the 112 subjects capable of treadmill exercise.
Patients with a large total or ischemic PDS were older, less likely to be able to perform exercise treadmill stress and more likely to have accompanying LV dysfunction ( Table 2 ). There was no difference in the mean number of cardiac risk factors between subjects with a normal SPECT result and those with a large perfusion defect.
| Variable | Normal (n = 151) | PDS <15% (n = 25) | PDS ≥15% (n = 18) | p Value ∗ | IPDS <10% (n = 30) | IPDS ≥10% (n = 13) | p Value † |
|---|---|---|---|---|---|---|---|
| Age | 56.0 ± 10.2 | 53.9 ± 10.7 | 62.8 ± 7.3 | 0.01 | 54.8 ± 10.0 | 65.0 ± 8.1 | <0.01 |
| Male | 88 (58%) | 20 (80%) | 8 (44%) | 0.046 | 22 (73%) | 7 (54%) | 0.27 |
| Diabetes mellitus | 46 (31%) | 15 (60%) | 7 (39%) | 0.02 | 10 (33%) | 7 (54%) | 0.22 |
| Hypertension | 124 (82%) | 21 (84%) | 13 (72%) | 0.56 | 26 (87%) | 8 (64%) | 0.14 |
| Hypercholesterolemia | 104 (69%) | 17 (68%) | 12 (67%) | 0.98 | 20 (67%) | 9 (73%) | 0.97 |
| Smoker | 71 (47%) | 11 (44%) | 8 (44%) | 0.95 | 15 (50%) | 5 (36%) | 0.78 |
| Number of risk factors | 2.3 ± 0.1 | 2.4 ± 1.0 | 2.4 ± 1.4 | 0.55 | 2.4 ± 1.0 | 2.3 ± 1.5 | 0.66 |
| Duke pre-test probability (%) | 6 (3 – 8) | 5 (3 – 8) | 7 (3 – 18) | 0.01 | 4 (3 – 7) | 10 (4 – 20) | 0.02 |
| Symptomatic chest pain | 35 (23%) | 8 (32%) | 5 (28%) | 0.61 | 8 (27%) | 5 (36%) | 0.46 |
| Ability to perform exercise stress | 96 (64%) | 11 (44%) | 5 (28%) | <0.01 | 13 (43%) | 3 (23%) | <0.01 |
| LV ejection fraction (%) | 57 (51 – 63) | 55 (50 – 60) | 46 (29 – 51) | <0.001 | 51 (45 – 57) | 50 (34 – 61) | <0.001 |
∗ Normal SPECT versus total PDS <15%, total PDS ≥15%.
As depicted in Table 3 , subjects with a higher CACS were older and more frequently men and diabetic. There was a graded association between increasing CACS and worsening LV function. There was no significant association between the frequency of symptomatic chest pain and CACS severity.
| Variable | CACS Severity Groups (n = 194) | ||||
|---|---|---|---|---|---|
| 0 – 10 (n = 68) | 11 – 100 (n = 39) | 101 – 400 (n = 35) | >400 (n = 52) | P Value | |
| Age (years) | 51.8 ± 11.5 | 58.5 ± 7.6 | 58.1 ± 7.9 | 59.1 ± 9.6 | <0.001 |
| Male | 29 (43%) | 26 (67%) | 21 (60%) | 42 (79%) | <0.001 |
| Diabetes mellitus | 15 (22%) | 12 (31%) | 18 (51%) | 19 (36%) | 0.02 |
| Hypertension | 59 (87%) | 33 (85%) | 26 (74%) | 42 (79%) | 0.44 |
| Hypercholesterolemia | 46 (68%) | 25 (64%) | 25 (71%) | 37 (70%) | 0.88 |
| Smoker | 29 (43%) | 22 (56%) | 15 (43%) | 24 (45%) | 0.55 |
| Number of risk factors | 2.2 ± 1.0 | 2.4 ± 1.0 | 2.5 ± 1.0 | 2.3 ± 1.0 | 0.50 |
| Duke pre-test probability (%) | 4 (2 – 5) | 5 (4 – 8) | 5 (3 – 8) | 7 (4 – 9) | 0.06 |
| Symptomatic chest pain | 24 (35%) | 11 (28%) | 7 (20%) | 8 (15%) | 0.07 |
| Ability to perform exercise stress | 44 (65%) | 23 (59%) | 20 (57%) | 26 (49%) | 0.45 |
| LV ejection fraction (%) | 57 (54 – 64) | 58 (49 – 62) | 57 (48 – 64) | 53 (44 – 59) | 0.047 |
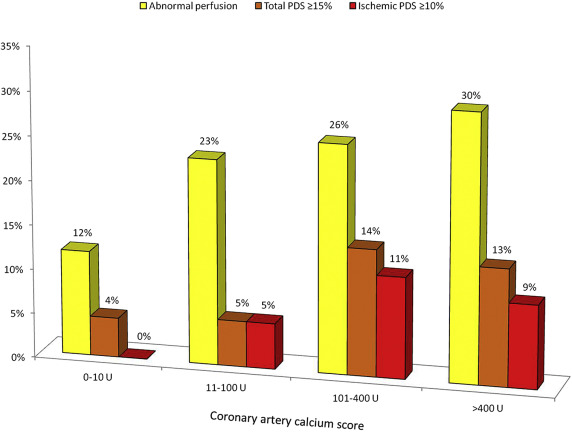
Subjects with a normal SPECT result had a lower median Agatston score compared to those with abnormal perfusion (35 U [IQR 0 to 349 U] vs 306 U [IQR 14 to 912 U]; p <0.01). There was a weak-graded association between the increasing proportion of patients with abnormal perfusion and increasing CACS severity (p = 0.049; Figure 2 ). There was, however, no significant association between CACS severity and the frequency of a large stress-induced total (≥10%) or ischemic (≥10%) PDS. An abnormal SPECT result was observed in 12% of subjects (8 of 68) with a CACS 0 to 10 and in 23% of subjects (9 of 39) with a CACS 11 to 100 U. In 4% of patients with only minimal CACS (3 of 68), a high-risk SPECT profile was demonstrated based on the stress-induced total PDS.