The prognostic significance of postprocedure sustained ventricular tachycardia or ventricular fibrillation (VT/VF) in patients undergoing primary percutaneous coronary intervention (PPCI) for ST-segment elevation myocardial infarction (STEMI) has rarely been studied, although a previous study has suggested that its occurrence portends decreased survival. We examined outcomes from the prospective large-scale multicenter randomized HORIZONS-AMI trial to evaluate the incidence, clinical correlates, and outcomes of in-hospital sustained VT/VF after PPCI. Of 3,485 patients undergoing PPCI in whom VT/VF did not occur before or during the procedure, 181 patients (5.2%) developed VT/VF after PPCI. Most postprocedural VT/VF episodes (85%) occurred in the first 48 hours. Patients with postprocedural VT/VF were more likely men with Killip class >I on presentation but had a lower prevalence of hypertension and diabetes. Patients with postprocedural VT/VF were also less frequently taking β blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers at admission. Mean door-to-balloon time was shorter and Thrombolysis In Myocardial Infarction grade 0 flow before PPCI was more common in patients with VT/VF, although Thrombolysis In Myocardial Infarction grade 3 flow rates after PPCI did not vary. There were no significant differences in adjusted 3-year rates of mortality (hazard ratio 0.73, 95% confidence interval 0.30 to 1.79) or composite major adverse clinical events (death, myocardial infarction, target vessel revascularization, or stroke; hazard ratio 0.71, 95% confidence interval 0.44 to 1.15) in patients with versus without postprocedural sustained VT/VF. In conclusion, sustained VT/VF after PPCI in the HORIZONS-AMI trial was not significantly associated with 3-year mortality or major adverse clinical events. Further studies are required to address the prognostic significance of VT/VF in patients with STEMI undergoing PPCI.
Primary percutaneous coronary intervention (PPCI) is the preferred mode of reperfusion for patients with ST-segment elevation myocardial infarction (STEMI). A growing body of knowledge accumulated over the previous 2 decades has informed the risk stratification of patients with STEMI undergoing PPCI. However, the impact of sustained ventricular tachycardia or ventricular fibrillation (VT/VF), the most common reason for close in-hospital cardiac monitoring in patients with STEMI, on the long-term prognosis of patients undergoing PPCI has not been well elucidated. Two studies have evaluated the prognostic significance of VT/VF occurring during or after PPCI and have provided conflicting results. The Primary Angioplasty in Myocardial Infarction (PAMI) trial reported no significant impact of VT/VF occurring during cardiac catheterization on subsequent survival, whereas the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial indicated a strong association with VT/VF during or after PPCI and subsequent mortality. These findings have not been validated in other studies. We therefore examined the data from the large-scale prospective multicenter randomized Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial to evaluate the incidence, clinical correlates, and outcomes of patients undergoing PPCI who had postprocedural in-hospital VT/VF.
Methods
The design and results of the HORIZONS-AMI trial have been described previously. In brief, HORIZONS-AMI was a prospective open-label 2 × 2 factorial randomized multicenter trial of 3,602 patients enrolled at 123 international centers. Patients were randomized 1:1 in the emergency room to anticoagulation with unfractionated heparin plus routine use of a glycoprotein IIb/IIIa inhibitor or bivalirudin alone, with bail-out glycoprotein IIb/IIIa inhibitor use reserved for refractory no-reflow or large thrombus. After angiography patients with lesions eligible for stenting then underwent a second randomization (3:1) to Taxus Express-2 (Boston Scientific, Natick, Massachusetts) paclitaxel-eluting stents or identical Express-2 bare-metal stents. Aspirin was administered daily during the index hospitalization and prescribed indefinitely after discharge. The initial loading dose of thienopyridine was left to the investigator’s discretion, although a clopidogrel ≥300-mg loading dose was required according to the trial protocol. For our investigation, we excluded 117 patients (3.2%) who had VT/VF before or during the cardiac catheterization. The remaining 3,485 patients formed the basis of this analysis.
The primary end-point definitions have been previously detailed. Major adverse cardiovascular events (MACEs; composite of all-cause death, stroke, reinfarction, and unplanned revascularization for ischemia) and major bleeding were adjudicated by an independent clinical events committee blinded to treatment assignment. Sustained VT was predefined according to the protocol as wide complex tachycardia not related to bundle branch block at a rate >100 beats/min and lasting for >30 seconds or that required cardioversion/defibrillation. In the trial date of VT/VF was collected but not the actual time of the day it occurred. Sites were required to mark on the case-report form whether the arrhythmia occurred during or after the procedure. Duration of telemetric monitoring after PPCI was not specified in the trial.
Patients were categorized by presence or absence of sustained VT/VF after PPCI. Continuous variables were expressed as median and interquartile range and were compared using Wilcoxon rank-sum test. Categorical variables were compared using the chi-square test or Fisher’s exact test (when the expected cell count was <5). Event analyses were performed using time-to-event data (for which patients were censored at the time of withdrawal from the study or at last follow-up), are displayed using Kaplan–Meier plots, and were compared using log-rank test. Logistic regression was used to identify the independent clinical correlates of VT/VF. The multivariable model was built by stepwise variable selection with entry and exit criteria set at a p value ≤0.20. Clinically relevant patient-level candidate predictors were selected to prevent model overfitting and included age, gender, hypertension, diabetes, baseline Killip class, baseline heart rate, systolic blood pressure, sum of ST-segment elevations, hemoglobin, creatinine clearance, white blood cell count, Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 or I (vs II or III), symptom-to-balloon time, left ventricular ejection fraction, multivessel coronary artery disease, postprocedural TIMI flow grade 3 (vs 0 to 2), baseline thrombus, prerandomization β blockers, and randomized treatment assignment (bivalirudin vs heparin plus glycoprotein IIb/IIIa inhibitor). Multivariable Cox proportional hazard models were used to derive the adjusted hazard ratio (HR) and 95% confidence interval (CI) of VT/VF for 3-year rates of mortality and MACEs to ascertain the independent prognostic significance of VT/VF on these events. All statistical tests were 2-tailed. Statistical significance was set at a level of 0.05.
Results
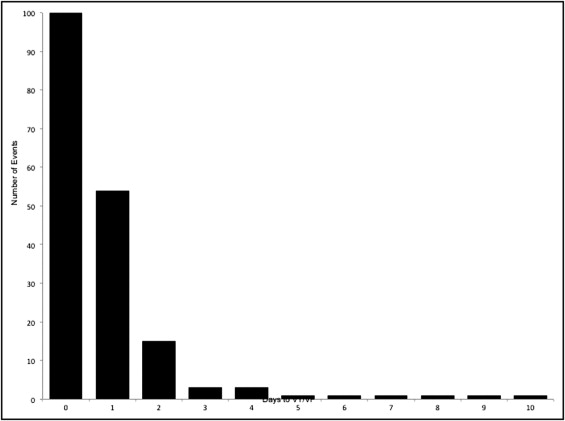
Of the 3,485 patients included in this study, in-hospital VT/VF after PPCI occurred in 181 patients (5.2%). Most of these events occurred early (85% within first 48 hours; Figure 1 ) . Median day of occurrence of postprocedural VT/VF was day 0, i.e., the day of PPCI (interquartile range 0 to 1). Demographic and clinical features in patients with and without VT/VF were similar for the most part with a few exceptions ( Table 1 ). Patients with VT/VF were more likely to be men and present with Killip class >I, whereas hypertension and diabetes were less prevalent in patients with VT/VF. Patients with VT/VF had shorter symptom-to-balloon and door-to-balloon times, higher prevalence of TIMI grade 0 flow before PPCI, and higher incidence of single-vessel PCI and stent use ( Table 2 ). Use of an aspiration or thrombectomy device was significantly greater in the VT/VF group. Use of most cardiac medications in hospital and at discharge did not differ significantly between the 2 groups except for a lower use of β blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers before randomization and a lower prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at discharge in patients with postprocedural VT/VF ( Table 3 ). Amiodarone was used in a minority and given more frequently at discharge in patients with VT/VF (5.6% vs 2.6%, p = 0.03). Factors independently associated with increased risk of VT/VF after PPCI are listed in Table 4 .
| Characteristic | VT/VF | p Value | |
|---|---|---|---|
| No (n = 3,304) | Yes (n = 181) | ||
| Age (years) ⁎ | 60 (52–70) | 59 (51–68) | 0.29 |
| Women | 784/3,304 (24%) | 30/181 (17%) | 0.03 |
| Weight (kg) ⁎ | 80 (70–90) | 82 (73–94) | 0.09 |
| Whites | 3,096/3,304 (94%) | 169/181 (94%) | 0.86 |
| Hypertension | 1,783/3,301 (54%) | 75/181 (41%) | 0.001 |
| Diabetes mellitus | 556/3,301 (17%) | 15/181 (8.3%) | 0.003 |
| Current smoker | 1,499/3,285 (46%) | 88/180 (49%) | 0.39 |
| History of hyperlipidemia | 1,424/3,301 (43%) | 74/181 (41%) | 0.55 |
| Previous myocardial infarction | 361/3,301 (11%) | 14/181 (7.7%) | 0.18 |
| Previous percutaneous coronary interventions | 353/3,300 (11%) | 16/181 (8.8%) | 0.43 |
| Previous coronary bypass | 93/3,301 (2.8%) | 5/181 (2.8%) | 0.97 |
| Previous congestive heart failure | 93/3,301 (2.8%) | 6/181 (3.3%) | 0.69 |
| Peripheral vascular disease | 146/3,300 (4.4%) | 7/181 (3.9%) | 0.72 |
| Renal insufficiency | 98/3,300 (3.0%) | 2/181 (1.1%) | 0.14 |
| Ventricular tachycardia/fibrillation | 22/3,301 (0.7%) | 0/181 (0.0%) | 0.63 |
| Previous defibrillator implantation | 0 | 0 | — |
| Atrial fibrillation | 63/3,307 (1.9%) | 3/181 (1.7%) | 1.00 |
| Presenting characteristics | |||
| Heart rate (beats/min) ⁎ | 76 (65–88) | 72 (64–82) | 0.01 |
| Systolic blood pressure (mm Hg) ⁎ | 137 (120–155) | 130 (116–145) | 0.004 |
| Infarct location: anterior | 1,336/2,914 (46%) | 76/172 (44%) | |
| Infarct location: nonanterior | 1,578/2,914 (54%) | 96/172 (56%) | |
| Killip class | |||
| I | 3,033/3,294 (92%) | 154/181 (85%) | 0.0009 |
| II | 211/3,294 (6.4%) | 23/181 (13%) | 0.001 |
| III | 28/3,294 (0.9%) | 2/181 (1.1%) | 0.67 |
| IV | 22/3,294 (0.7%) | 2/181 (1.1%) | 0.36 |
| Sum of ST-segment deviations ⁎ | 7.1 (4.2–12.0) | 7.5 (4.6–11.7) | 0.49 |
| Hematocrit (%) ⁎ | 43 (40–45) | 43 (40–46) | 0.22 |
| Creatinine clearance (ml/min) ⁎ | 89 (68–114) | 88 (72–110) | 0.93 |
| Characteristic | VT/VF | p Value | |
|---|---|---|---|
| No (n = 3,304) | Yes (n = 181) | ||
| Symptom onset-to-balloon time (min) ⁎ | 225 (162–339) | 195.0 (149–295) | 0.002 |
| Door-to-balloon time (min) ⁎ | 100 (74–136) | 88 (68–120) | 0.005 |
| Preprocedural Thrombolysis In Myocardial Infarction grade flow | |||
| 0 | 1,812/3,273 (55%) | 121/180 (67%) | 0.002 |
| 1 | 290/3,273 (8.9%) | 18/180 (10%) | 0.60 |
| 2 | 533/3,273 (16%) | 21/180 (12%) | 0.10 |
| 3 | 638/3,273 (20%) | 20/180 (11%) | 0.005 |
| Postprocedural Thrombolysis In Myocardial Infarction grade flow | |||
| 0 | 56/3,278 (1.7%) | 0/180 (0.0%) | 0.12 |
| 1 | 23/3,278 (0.7%) | 1/180 (0.6%) | 1.00 |
| 2 | 204/3,278 (6.2%) | 9/180 (5.0%) | 0.51 |
| 3 | 2,995/3,278 (91%) | 170/180 (94%) | 0.15 |
| Infarct-related coronary artery | |||
| Left main | 20/3,284 (0.6%) | 0/180 (0.0%) | 0.62 |
| Left anterior descending | 1,337/3,284 (41%) | 73/180 (41%) | 0.97 |
| Left circumflex | 527/3,284 (16%) | 22/180 (12%) | 0.17 |
| Right | 1,370/3,284 (42%) | 83/180 (46%) | 0.24 |
| Saphenous vein graft | 28/3,284 (0.9%) | 2/180 (1.1%) | 0.67 |
| Left ventricular ejection fraction (%) ⁎ | 60 (51–68) | 60 (48–68) | 0.59 |
| Single-vessel percutaneous coronary intervention | 2,857/2,984 (95.8%) | 170/172 (98.8%) | 0.046 |
| Stent usage | 2,856/3,061 (93.3%) | 168/173 (97.1%) | 0.048 |
| Peak activated clotting time (min) ⁎ | 308 (249–384) | 317 (250–400) | 0.56 |
| Femoral approach | 3,078/3,299 (93.3%) | 178/181 (98.3%) | 0.007 |
| Closure device used | 807/2,649 (28.3%) | 68/170 (40.0%) | 0.001 |
| Any thrombectomy device used | 24/3,010 (0.8%) | 7/170 (4.1%) | 0.001 |
| Any aspiration catheter used | 325/3,018 (10.8%) | 36/172 (20.9%) | <0.0001 |
| Distal embolization or side branch closure | 7/1,645 (0.4%) | 1/92 (1.1%) | 0.35 |
| ST-segment resolution ≤70% | 1,315/2,562 (51.3%) | 73/154 (47.4%) | 0.34 |
| Medical Therapies | VT/VF | p Value | |
|---|---|---|---|
| No (n = 3,304) | Yes (n = 181) | ||
| Pre-enrollment medications | |||
| β Blockers | 725/3,296 (22%) | 26/181 (14%) | 0.02 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 797/3,296 (24%) | 30/181 (17%) | 0.02 |
| Amiodarone | 15/3,297 (0.5%) | 0/181 (0.0%) | 1.00 |
| In-hospital treatment | |||
| Aspirin | 3,288/3,297 (100%) | 181/181 (100%) | 1.00 |
| Thienopyridine agents | 3,243/3,296 (98%) | 180/181 (99%) | 0.53 |
| β Blockers | 3,012/3,304 (91%) | 164/181 (91%) | 0.80 |
| Preprocedure heparin | 2,336/3,297 (71%) | 130/181 (72%) | 0.78 |
| Heparin in catheter laboratory | 1,667/3,296 (51%) | 91/181 (50%) | 0.94 |
| Bivalirudin in catheter laboratory | 1,606/3,288 (49%) | 91/179 (51%) | 0.60 |
| Glycoprotein IIbIIIa antagonists | 1,789/3,292 (54%) | 98/181 (54%) | 0.96 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker | 2,675/3,293 (81%) | 137/181 (76%) | 0.06 |
| Statins | 3,043/3,299 (92%) | 167/181 (92%) | 0.95 |
| Discharge medications | |||
| Aspirin | 3,140/3,219 (98%) | 177/178 (99%) | 0.13 |
| Thienopyridine agents | 2,993/3,221 (93%) | 172/178 (97%) | 0.06 |
| β Blockers | 2,922/3,284 (89%) | 157/181 (87%) | 0.35 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker | 2,614/3,221 (81%) | 132/178 (74%) | 0.02 |
| Statins | 3,018/3,221 (94%) | 169/178 (95%) | 0.50 |
| Amiodarone | 85/3,221 (2.6%) | 10/178 (5.6%) | 0.03 |
| Outcomes | VT/VF | p Value | |
|---|---|---|---|
| No (n = 3,304) | Yes (n = 181) | ||
| 30-Day events | |||
| Net adverse clinical events ⁎ | 362/3,304 (11%) | 19/181 (11%) | 0.83 |
| Major adverse cardiac events † | 179/3,304 (5.4%) | 8/181 (4.4%) | 0.56 |
| Death | 85/3,304 (2.6%) | 4/181 (2.2%) | 0.75 |
| Cardiac | 78/3,304 (2.4%) | 2/181 (1.1%) | 0.27 |
| Noncardiac | 7/3,304 (0.2%) | 2/181 (1.1%) | 0.02 |
| Recurrent myocardial infarction | 58/3,304 (1.8%) | 2/181 (1.1%) | 0.50 |
| Death or recurrent myocardial infarction | 134/3,304 (4.1%) | 5/181 (2.8%) | 0.38 |
| Target vessel revascularization | 75/3,304 (2.3%) | 3/181 (1.7%) | 0.58 |
| Target lesion revascularization | 69/3,304 (2.1%) | 3/181 (1.7%) | 0.68 |
| Stent thrombosis | 67/3,304 (2.3%) | 3/171 (1.8%) | 0.63 |
| Major bleeding not related to coronary artery bypass grafting | 229/3,310 (7.0%) | 12/181 (6.6%) | 0.87 |
| Major bleeding related to coronary artery bypass grafting | 294/3,310 (9.0%) | 18/181 (10) | 0.63 |
| Red blood cell transfusion | 99/3,304 (3.0%) | 5/181 (2.8%) | 0.85 |
| Stroke | 21/3,304 (0.6%) | 5/181 (2.8%) | 0.001 |
| Transient ischemic attack | 3/3,310 (0.1%) | 1/181 (0.6%) | 0.08 |
| 3-Year events | |||
| Net adverse clinical events ⁎ | 847/3,304 (27%) | 38/181 (22%) | 0.18 |
| Major adverse cardiac events † | 692/3,304 (22%) | 29/181 (17%) | 0.12 |
| Death | 214/3,304 (6.7%) | 8/181 (4.6%) | 0.27 |
| Cardiac | 126/3,304 (3.9%) | 4/181 (2.3%) | 0.27 |
| Noncardiac | 88/3,304 (2.9%) | 4/181 (2.3%) | 0.70 |
| Recurrent myocardial infarction | 214/3,304 (7.0%) | 11/181 (6.5%) | 0.78 |
| Death or recurrent myocardial infarction | 406/3,304 (13%) | 16/181 (9.2%) | 0.17 |
| Target vessel revascularization | 416/3,304 (14%) | 21/181 (12%) | 0.64 |
| Target lesion revascularization | 326/3,304 (11%) | 19/181 (11%) | 0.83 |
| Stent thrombosis | 158/3,304 (5.6%) | 11/181 (6.7%) | 0.59 |
| Major bleeding not related to coronary artery bypass grafting | 281/3,304 (8.7%) | 14/181 (7.9%) | 0.70 |
| Major bleeding related to coronary artery bypass grafting | 348/3,304 (11%) | 19/181 (11%) | 0.98 |
| Transfusion | 138/3,310 (4.3%) | 6/181 (3.4%) | 0.57 |
| Stroke | 56/3,304 (1.8%) | 7/181 (4.0%) | 0.04 |
| Transient ischemic attack | 10/3,304 (0.3%) | 2/181 (1.2%) | 0.08 |
| 30-Day to 3-year events | |||
| Net adverse clinical events ⁎ | 564/3,304 (19%) | 25/181 (15%) | 0.25 |
| Major adverse cardiac events † | 537/3,304 (18%) | 22/181 (13%) | 0.15 |
| Death | 129/3,304 (4.3%) | 4/181 (2.4%) | 0.25 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


