Previous studies of patients with stable coronary artery disease have demonstrated that decreases in the left ventricular ejection fraction (LVEF) during acute mental stress are predictive of adverse clinical outcomes. The aim of the present study was to examine the prospective relation of mental stress on clinical outcomes in a sample of 138 patients with stable coronary artery disease. Patients underwent mental stress testing and were followed for a median of 5.9 years to assess the occurrence of the combined end point of myocardial infarction or all-cause mortality. There were 32 events (17 nonfatal myocardial infarctions and 15 deaths) over the follow-up period. Of the 26 patients who exhibited myocardial ischemia during mental stress testing, 11 (42%) sustained subsequent clinical events, compared to 21 of the 112 patients (19%) who showed no mental stress–induced ischemia. LVEF change during mental stress was also related to the clinical events in a graded, continuous fashion, with each 4% decrease from the LVEF at rest associated with an adjusted hazard ratio of 1.7, (95% confidence interval 1.1 to 2.6, p = 0.011). In conclusion, reductions in the LVEF during mental stress are prospectively associated with adverse clinical outcomes.
A decrease in the left ventricular ejection fraction (LVEF) during mental stress (LVEF-MS) has been shown to identify patients at increased risk for adverse clinical events among those with documented coronary artery disease (CAD). Previously, we showed that reductions in the LVEF in response to mental stress were associated with an increased likelihood of subsequent clinical events, but these events were primarily revascularization procedures. Because revascularization procedures may not always reflect extraclinical factors as well as disease progression, we reexamined this association using “hard” end points of all-cause death and myocardial infarction (MI) in a sample of patients with stable CAD.
Methods
Data were available from baseline assessments of 138 of 144 patients with documented CAD who participated in a clinical trial of exercise and behavioral stress management. To participate in the trial, patients had to have documented CAD (by previous MI, coronary artery bypass graft surgery, coronary angioplasty, and/or ≥75% stenosis in ≥1 major coronary artery) and a positive results on treadmill exercise testing within the previous year. Informed consent was obtained for all participants, and the study protocol was approved by the Institutional Review Board at Duke University Medical Center.
Unless medically contraindicated, patients were briefly withdrawn from anti-ischemic medications (e.g., β blockers, calcium channel blockers, and long-acting nitrates) ≥48 hours before testing; the medication washout period was ≥5 half-lives of the anti-ischemic medication. Twenty-eight patients were unable to be withdrawn from their medications and were tested on their usual doses of anti-ischemic medications. After a 40-minute rest period, mental stress testing was performed, in which patients were presented with 2 mental stress tasks, public speaking and mirror trace, in counterbalanced order. The speaking stressor required participants to give a speech on a controversial current events topic after 1 minute of preparation. The mirror trace stressor required participants to outline the shape of a star from its reflection in a mirror. Each task lasted 5 minutes, with a 10-minute rest period between each stressor. These tasks have been used in previous work and have been found to elicit ischemia in vulnerable patients.
To determine the presence of myocardial ischemia, R-wave-synchronized, gated equilibrium radionuclide ventriculography using Paragon PBR software (Medasys, Inc., Ann Arbor, Michigan) was performed before and during each stressor at 20 frames/cardiac cycle using a gamma camera (Siemens Gammasonics, Inc., Des Plaines, Illinois) equipped with a sodium iodide crystal and an all-purpose collimator. Images were obtained after the labeling of autologous red blood cells with technetium-99m pertechnetate using the in vivo technique. Imaging was conducted during the last 2 minutes of the rest period, the first minute of speech preparation, and at 2 and 4 minutes for the speaking and mirror trace stressors with the camera in the left anterior oblique view. The LVEF was determined using the PBR software.
We defined LVEF-MS as the change from the LVEF at rest, averaged across the speaking and trace tasks. We also classified patients who had reductions in the LVEF of ≥5% (e.g., from 55% during rest to an average of ≤50% during the tasks) as exhibiting mental stress–induced myocardial ischemia. Participants were assessed for clinical events 4 months after testing and annually thereafter through a combination of telephone and mail contact with participants, examination of medical records, and public sources of vital statistics. The primary outcome was the combined end point of all-cause mortality or MI.
We used multivariate Cox proportional-hazards models to estimate the hazard associated with LVEF-MS, adjusting for age, gender, history of MI, and LVEF at rest. LVEF-MS was modeled as a continuous variable and scaled such that the resulting hazard ratio (HR) represented the change in hazard for every 4% (the interquartile range of LVEF-MS) reduction in LVEF-MS. The LVEF at rest and age also were modeled as continuous variables scaled to their interquartile ranges (14% and 15 years, respectively). We examined the association between the continuous LVEF-MS measure and the end point for possible nonlinearity using a flexible nonparametric curve-fitting algorithm. We also conducted a number of sensitivity analyses, adjusting for further potential confounders, including revascularization procedures that occurred during follow-up (modeled as a time-varying covariate). In addition, we evaluated whether the relation between LVEF-MS and the combined end point differed for patients who were tapered off their cardiac medications during the mental stress testing and those who were still taking their medications by testing an interaction term for LVEF-MS by medication status (on vs off medication) in the Cox model. Finally, we reestimated the primary Cox models for the separate end points of death and MI.
Results
One hundred thirty-eight participants (98%) had adequate radionuclide ventriculographic studies during mental stress testing. The average age of the sample was 62 years, with most being white men. Twenty-six patients (19%) of the sample exhibited mental stress–induced ischemia. Patients with mental stress–induced ischemia were more likely to belong to ethnic minorities, to report histories of diabetes, and to have lower serum high-density lipoprotein levels at the time of testing compared to the nonischemic patients ( Table 1 ). The median follow-up time was 5.9 years (interquartile range 4.8 to 7.2 years, range 35 days to 8.8 years). There were 18 deaths and 17 nonfatal MIs. Of the 18 deaths, 4 were known to be related to cardiac causes, 2 were known to be noncardiac, and 12 were of unknown causes. In 3 cases, death was preceded by MI, so the initial MI rather than death was used as the outcome. Thus, 32 unique events (15 deaths and 17 MIs) were available for analysis.
| Variable | No Ischemia | Ischemia | All Participants | p Value (Ischemia vs No Ischemia) ⁎ |
|---|---|---|---|---|
| (n = 112) | (n = 26) | (n = 138) | ||
| Age (years) | 62.5 (55.8 to 71.2) | 60.0 (51.2 to 69.0) | 62.0 (55.0 to 70.0) | 0.471 |
| Men | 79 (71%) | 17 (65%) | 96 (70%) | 0.607 |
| Ethnicity | 0.030 † | |||
| African America | 17 (15%) | 8 (31%) | 25 (18%) | |
| Caucasian | 91 (81%) | 15 (58%) | 106 (77%) | |
| Other ethnicity | 4 (4%) | 3 (12%) | 7 (5%) | |
| Education | 0.391 † | |||
| High school or less | 32 (29%) | 7 (27%) | 39 (28%) | |
| Some college | 39 (35%) | 6 (23%) | 45 (33%) | |
| College or more | 41 (37%) | 13 (50%) | 54 (39%) | |
| Body mass index (kg/m 2 ) | 28.4 (25.8 to 32.3) | 30.1 (25.5 to 32.4) | 28.9 (25.7 to 32.3) | 0.584 |
| Hypertension | 60 (54%) | 15 (58%) | 75 (54%) | 0.704 |
| Diabetes mellitus | 21 (19%) | 10 (38%) | 31 (22%) | 0.030 |
| Current smokers | 14 (12%) | 3 (12%) | 17 (12%) | 0.893 |
| Quit smoking | 72 (64%) | 15 (58%) | 87 (63%) | 0.530 |
| Past myocardial reinfarction | 63 (56%) | 15 (58%) | 78 (57%) | 0.894 |
| Past revascularization | 50 (45%) | 12 (46%) | 62 (45%) | 0.889 |
| Total cholesterol (mg/dl) | 180 (160 to 205) | 183 (166 to 197) | 180 (161 to 201) | 0.819 |
| Low-density lipoprotein (mg/dl) | 97 (87,124) | 114 (88,125) | 98 (87,125) | 0.539 |
| High-density lipoprotein (mg/dl) | 45 (39.54) | 40 (35.47) | 44 (38.52) | 0.016 |
| Triglycerides (mg/dl) | 141 (102 to 211) | 138 (100 to 166) | 140 (102 to 210) | 0.888 |
| β blockade | 81 (72%) | 18 (72%) | 99 (72%) | 0.974 |
| Nitrates | 34 (30%) | 8 (23%) | 42 (31%) | 0.872 |
| Calcium channel blockade | 25 (22%) | 5 (20%) | 30 (22%) | 0.800 |
| Anticoagulants | 102 (91%) | 22 (85%) | 124 (90%) | 0.326 |
| Statins | 87 (78%) | 20 (77%) | 107 (78%) | 0.934 |
| LVEF at rest (%) | 57.5 (51.0 to 66.2) | 56.2 (51.1 to 63.0) | 57.2 (51.0 to 64.9) | 0.729 |
| Reduction in LVEF during mental stress (%) | −0.75 (−2.25 to 0.75) | −6.50 (−7.19 to −5.56) | −1.50 (−3.50 to 0.50) | <0.001 |
| Medication during testing | 21 (19%) | 7 (27%) | 28 (20%) | 0.351 |
⁎ Wilcoxon’s test was used for continuous variables, and Pearson’s chi-square test was used for categorical variables.
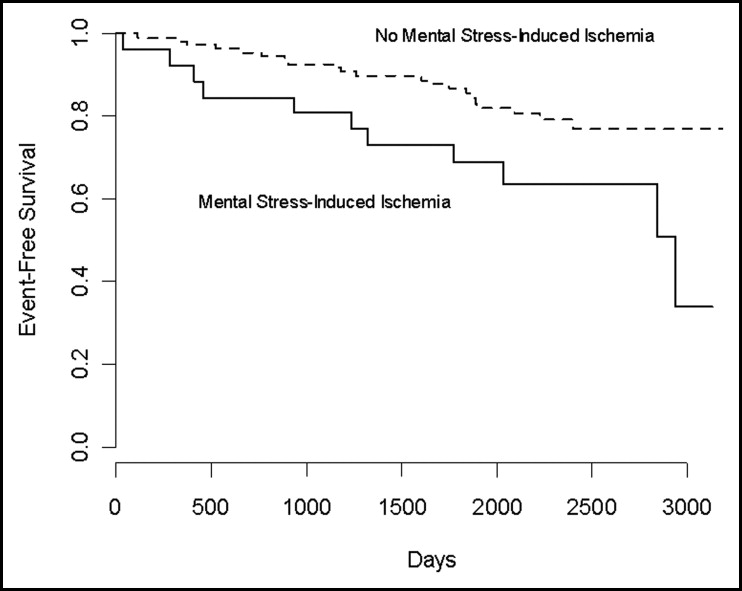
Among the 26 patients who exhibited mental stress–induced ischemia, 42% (n = 11) sustained clinical events during the follow-up period, compared with 19% (n = 21) of the 112 patients who showed no ischemia. Figure 1 shows the unadjusted Kaplan-Meier curves for patients with and without mental stress–induced ischemia. The log-rank test comparing the 2 curves was statistically significant (p = 0.020). Turning to the Cox regression results, LVEF-MS was significantly associated with the time to the combined end point (see Table 2 ), adjusting for age, gender, previous MI, and the LVEF at rest. We found no evidence that the association between LVEF-MS and the combined end point was nonlinear (p = 0.485).