Fig. 12.1
The anatomical relationships of the anterior leaflet of the mitral valve, including the right and left fibrous trigone, the non-coronary and left coronary cusps; and the subvalvular apparatus (Figure courtesy of Filsoufi F, Carpentier A. www.TheMitralValve.org)
Leaflets
The leaflet tissue consists of two leaflets—anterior and posterior; and two commissures (posteromedial and anterolateral). The commissures define the area where the anterior and posterior leaflets meet. The leaflets insert onto the entire circumference of the mitral annulus. The anterior mitral annulus has fibrous continuity with aortic annulus, and the left coronary cusp and half of the noncoronary cusp of the aortic valve (Figs. 12.1 and 12.2). The morphology of the anterior leaflet differs from the posterior leaflet in three other ways. The anterior (aortic) leaflet has a trapezoidal shape and it does not have scallops. It is attached to two-fifths of the annular circumference, and defines the boundary between the inflow and outflow tracts of the left ventricle. The posterior (mural) leaflet is attached to three-fifths of the annular circumference, and has a quadrangular shape divided into three scallops by two deep indentations. The leaflets have similar surface areas because the anterior leaflet is taller than the posterior leaflet. The atrial surface of the leaflets is divided into two zones: a proximal or smooth zone, which is normally thin and clear, and a distal rough zone. During systole the rough zones of the anterior and posterior leaflet come into apposition. The rough zone corresponds to the area of coaptation, and its ventricular surface is the site of insertion of most of the chordae tendinae (Fig. 12.3). A curved ridge, called the coaptation line, separates these two areas. Failure of coaptation leads to mitral regurgitation (Fig 12.4).
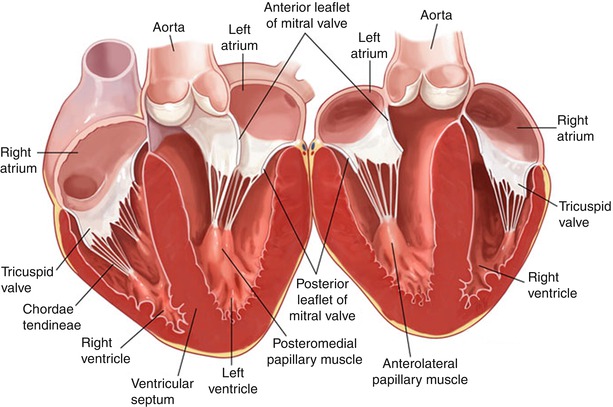
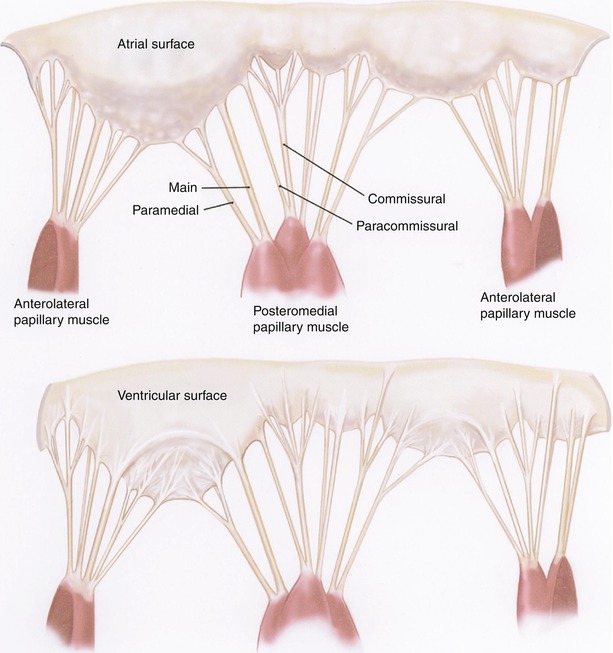

Fig. 12.2
Vertical cross section through the heart showing the fibrous continuity between the anterior mitral annulus and the left and half of the noncoronary cusp of the aortic valve, as well as the subvalvular apparatus (Figure courtesy of Filsoufi F, Carpentier A. www.TheMitralValve.org)

Fig. 12.3
The atrial surface of the leaflets are further divided into two zones, one peripheral smooth zone and one central rough zone which corresponds to the coaptation area, and which is the insertion site of most of the chordae tendineae (Figure courtesy of Carpentier et al. [8])
The anterolateral and posteromedial commissures can be identified by the axis of corresponding papillary muscles and the commissural chordae, which are fan-like in their distribution (Fig. 12.3). The free edge of the commissures, which occasionally take the form of a clearly defined commissural leaflet, is usually several millimeters from the annulus.
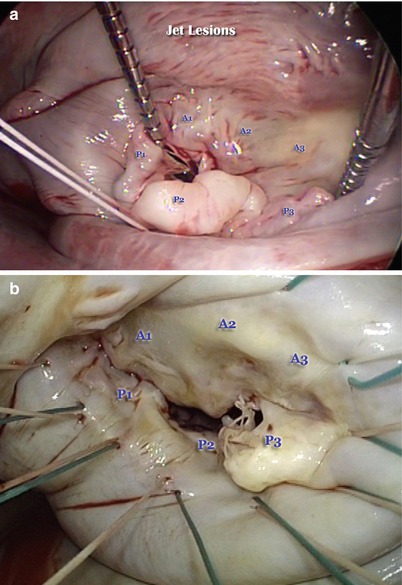
Segmental nomenclature is shown in Fig. 12.5. The three scallops of the posterior leaflet are identified as P1 (anterior scallop), P2 (middle scallop), and P3 (posterior scallop). P2 is usually the largest segment, P1 is commonly smaller than P3. The three corresponding segments of the anterior leaflet are A1 (anterior segment), A2 (middle segment), and A3 (posterior segment). The anterolateral and posteromedial commissures comprise the last two segments. This anatomical nomenclature allows precise location of valve pathology which may occur anywhere in the leaflet tissue.
Annulus
The mitral annulus is the saddle shaped fibrous junction between the left ventricle and atrium, with peaks located at the midpoint of the anterior and the posterior annulus. It is the site of insertion for the mitral valve leaflets and commissural tissue. The commissural annulus moves apically during systole, whereas the annular diameter narrows increasing the area of leaflet coaptation. The annulus lies several millimeters deep to the hinge point between the leaflets and left atrial tissue which is clearly seen when inspecting the valve through an atriotomy. The posterior mitral annulus consists of discontinous bands of fibrous tissues, and is not attached to any rigid structure so it is particularly prone to annular dilation, which can also affect the anterior portion of the mitral annulus between the two trigones, to a much lesser degree. The right fibrous trigone is where the fibrous annular tissue of the mitral, tricuspid, and noncoronary cusps of the aortic valve and the membranous septum coalesce, and the left fibrous trigone is formed by the junction of both left fibrous borders of the aortic and the mitral valve. Important anatomical structures in close proximity to the mitral annulus include the non-coronary and right coronary aortic sinuses, the circumflex coronary artery and the coronary sinus.
Sub-valvular Apparatus
The chordae tendinae are thin fibrous structures which connect the papillary muscles to the leaflets. They are classified according to the site of insertion. Marginal or primary chordae insert on the free margin of the leaflets and limit leaflet prolapse. The distance between marginal chordae in normal valves is usually less than 3–4 mm. Intermediate or secondary chordae insert onto the ventricular surface of the leaflets and reduce tension on the leaflet and primary chordae. The basal or tertiary chordae are attached to the posterior leaflet base connecting it to the mitral annulus.
Two papillary muscles give rise to chordae tendinae: each muscle supports both leaflets. The anterolateral papillary muscle, which usually has one head, arises between the mid and apical third of the lateral ventricular wall. The posteromedial papillary muscle has two heads, and arises from the middle third to apex of the posterior septal wall. The blood supply to the posteromedial papillary muscle comes from a single coronary territory: the right or the circumflex coronary artery depending on the coronary dominance. The anterolateral papillary muscle has a dual blood supply from the left anterior descending and the diagonal or marginal branches of the circumflex coronary arteries. The anterior papillary muscle is therefore less prone to acute ischemic rupture.
Functional Anatomy
During diastole the large surface area of the anterior mitral leaflet which opens into the left ventricle, divides the ventricle into the inflow chamber and the outflow tract. During the cardiac cycle the annulus contracts circumferentially, is displaced apically and folds in the intercommissural axis. This results in shortening of the distance between the annulus and left ventricular apex which augments the effects of ventricular systolic contraction, displacing blood into the aorta. During this phase of systole when the mitral valve is closed, there is normally a large surface of coaptation between the anterior and posterior leaflets preventing regurgitation. Dysfunction of any of the anatomical components of the mitral valve may reduce this surface of coaptation, leading to regurgitation. The likelihood of a competent and durable repair depends on the ability of the surgeon to identify and address any dysfunction.
Carpentier’s Pathophysiologic Triad
In order to help clinicians systematically describe regurgitant valves Carpentier developed a system of nomenclature which is widely used today, in which the disease etiology is described first (e.g., degenerative disease, endocarditis, rheumatic) then the primary lesion (e.g. chordal rupture, annular dilatation); and finally the resultant leaflet dysfunction [8]. This “pathophysiological triad” provides a particularly useful framework for surgical decision-making since etiology dictates long-term prognosis; valve dysfunction determines treatment strategy, and the individual lesions dictate surgical technique.
Carpentier’s Functional Classification
The Carpentier classification categorizes leaflet dysfunction into three types according to leaflet motion: Type I or normal motion, Type II or excessive leaflet motion, and Type III or restricted leaflet motion (Fig. 12.4) [9, 10]. There are two kinds of restricted leaflet motion: restricted leaflet opening (Type IIIa), commonly seen in rheumatic valve disease; and restricted leaflet closure (Type IIIb) most commonly seen in ischemic mitral valve regurgitation where ischemic ventricular dysfunction and dilatation results in lateral displacement of the posteromedial papillary muscle and chordal restriction of otherwise normal leaflets. Table 12.1 summarises the main lesions according to leaflet dysfunction, listing commonly associated lesions and selected reconstructive techniques used in robotic approaches. Carpentier’s system of nomenclature incorporates segmental valve anatomy, which assists systematic analysis of valve dysfunction and reporting of reconstructive techniques.
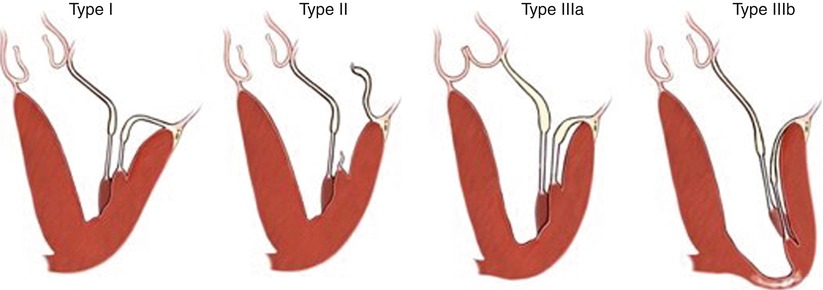

Fig. 12.4
Carpentier originally classified leaflet dysfunction into three types: Type I or normal motion, Type II or excessive leaflet motion, and Type III or restricted leaflet motion. Type III is subdivided into restricted opening (IIIa) and restricted closure (IIIb) (Figure courtesy of Carpentier et al. [8])
Table 12.1
Carpentier’s pathophysiologic triad of mitral regurgitation, composed of etiology, valve lesions and leaflet dysfunction, with specific surgical repair techniques, and possible percutaneous equivalent
Leaflet dysfunction | Type I | Type II | Type IIIa | Type IIIb | ||
|---|---|---|---|---|---|---|
Lesions | Annular dilatation | Leaflet perforations Clefts | Chordal elongation or rupture | Papillary muscle rupture | Leaflet thickening, commissural fusion, chordal fusion | Papillary muscle displacement, ventricular dilatation |
Etiology | Dilated cardiomyopathy | Endocarditis, occasionally trauma Congenital | Degenerative disease | Acute myocardial infarction | Rheumatic valve disease Occasionally carcinoid, radiation | Ischemic cardiomyopathy |
Reconstructive technique | Annuloplasty | Patch closure Primary closure | Quad. resection or chordal repair | Not suitable for robotic repair | Annuloplasty | |
Pathology
Degenerative Valve Disease
In developed countries the commonest pathology to directly involve the mitral valve leaflets is degenerative mitral valve disease. Degenerative mitral valve disease incorporates a spectrum of conditions in which infiltrative or dysplastic tissue changes cause elongation or rupture of the mitral valve chordae resulting in leaflet prolapse often associated with annular dilatation [9]. Fibroelastic deficiency occupies one end of the spectrum (Fig. 12.5). This is the second commonest cause of degenerative valve disease, and is commoner in older patients, who characteristically present with a short history of a symptomatic murmur. A family history is unusual. Fibroelastic deficiency is characterised by insufficient tissue in a normal sized valve: leaflets are thin and translucent, and chordae are flimsy and elongated. Regurgitation is most frequently caused by rupture of a single chord associated with a single thickened, prolapsing segment, usually P2, resulting in Carpentier Type II leaflet dysfunction. Occasionally bileaflet prolapse is seen. Secondary lesions at the level of the prolapsing segment with myxoid degeneration, excess tissue and leaflet thickening (Fig. 12.5a) may be confused with Barlow’s disease. Patients most commonly undergo surgery in their sixth decade or later.