High-energy blunt torso trauma
Multiple torso penetrations
Hemodynamic instability
Presenting coagulopathy and/or hypothermia
Major abdominal vascular injury with multiple visceral injuries
Multifocal or multi-cavity exsanguination with visceral injuries
Multiregional injury with competing priorities
When the decision to pursue damage control surgery has been made preoperatively, minimal additional evaluation in the emergency department is necessary prior to transporting the patient to the operating room. Time spent in the emergency room should be limited to establishment of a definitive airway and intravenous access and evaluation for both pneumothorax/massive hemothorax (via physical exam with or without chest radiography) and pericardial hemorrhage (via focused examination of the abdomen for trauma).
When made intraoperatively, the decision to perform damage control surgery is based broadly upon the six variables outlined in Table 4.2. After initial control of major bleeding and gastrointestinal contamination, an overall assessment of the patient’s metabolic and coagulation integrity is made by querying the following parameters: (1) hemodynamic status, (2) metabolic status, (3) temperature, (4) coagulation status, and (5) clinical assessment of nonsurgical bleeding. Hypotension from hemorrhagic shock is a clear indication to perform damage control. However, more subtle indicators of shock, such as increasing vasopressor requirements, hypocapnia, and metabolic acidosis, should all be evaluated. This task requires frequent and clear communication with the anesthesiology team. Patient temperature and coagulation status should be monitored frequently. Routine coagulation parameters, such as the activated partial thromboplastin time, prothrombin time, and international normalized ratio, are insensitive in detecting coagulopathy because they measure only the earliest stages of clot formation. Furthermore, results of these tests are typically not available immediately. For these reasons, we prefer point-of-care thromboelastography (TEG) [12]. Finally, the astute clinician will recognize the development of nonsurgical bleeding as evidenced by hemorrhage from raw surfaces, needle holes, and intravenous catheter sites. These findings signify the development of profound coagulopathy and mandate damage control surgery.
Table 4.2
Cases in which damage control should be considered intraoperatively
Indication | Example |
|---|---|
Inability to achieve hemostasis secondary to a recalcitrant coagulopathy | Massive transfusion with disseminated intravascular coagulation |
Inaccessible major venous injury | Retrohepatic vena caval disruption |
Anticipated need for a time-consuming procedure | Pancreaticoduodenectomy |
Demand for nonoperative control of extra-abdominal life-threatening injuries | Ruptured pelvic fracture hematoma requiring selective arterial embolization |
Inability to approximate the abdominal incision due to extensive splanchnic reperfusion-induced visceral edema | Protracted shock with massive fluid administration |
Desire to reassess abdominal contents | Extensive mesenteric venous injury |
Although the technical aspects of the abridged laparotomy are dictated by the injury pattern, in general, the initial damage control operation is divided into three sequential steps: (1) control of hemorrhage, (2) control of gastrointestinal contamination, and (3) temporary closure. Hemorrhage may be controlled by a variety of maneuvers, including packing, ligation, and shunting. In general, venous bleeding may be controlled by pressure pack tamponade using laparotomy pads. The packs are then left in place for transport to the ICU and removal at reoperation. By contrast, arterial or portal venous bleeding requires control with suture repair, ligation, or shunting. Under the extreme conditions of damage control, ligation of almost any vessel is compatible with life. If hemorrhage control can only be accomplished by manual pressure (e.g., sponge stick tamponade of an inferior vena cava injury prior to obtaining proximal and distal control), the surgeon should do so in order to allow for crystalloid and blood product resuscitation prior to exposing the patient to further hemorrhage. In extreme circumstances, control of hemorrhage may be possible only by cross-clamping of the abdominal aorta. Patients may be transported to the ICU with the clamp in place, provided that it is moved to an infrarenal location and that the cross-clamp time is monitored carefully, with flow being reestablished intermittently by transient release of the clamp. Control of gastrointestinal perforation is accomplished by rapid closure using either suture or staples. Although this phase of the operation should proceed rapidly, it should not be haphazard. Poorly placed sutures in tenuous areas such as the esophagus or duodenum may lead to catastrophic consequences subsequently provided that the patient survives, and the surgeon should take the extra few seconds to ensure proper suture placement through viable tissue and complete wound closure.
During the initial operation, efforts should be made to minimize both hypothermia and coagulopathy. Techniques to both prevent and reverse hypothermia include increasing the operating room temperature to >30 °C, infusing warmed fluids, covering body areas not in the operative field with warming devices, and using warmed irrigation fluid. Restoration of clotting function should be goal-directed using serial TEG tracings. When this method is not possible, blood component therapy should be replaced with a ratio of RBC/FFP/plts/cryoprecipitate of approximately 10:5:1:1 [13].
Stage II, Reassessment for Hemorrhage Control: An often underemphasized issue in the damage control literature involves the decision of when to transfer the patient from the operating room to the ICU. Although prompt transfer is both rational and cost-effective, premature departure with ongoing mechanical bleeding may lead to an inexorable bloody viscous cycle in the ICU. Finally, there are select cases in which packing may not be necessary and fascial closure is possible once coagulation integrity has been restored.
In cases of ongoing bleeding, a determination that the current amount of hemorrhage is “acceptable” as being nonsurgical must be made. Although this decision may be guided by the development of obvious nonsurgical bleeding (e.g., from intravenous sites), it is the authors’ contention that this decision is often made too late and the operation continued unnecessarily. One technique to aid in this determination is temporary (i.e., 20–30 min) abdominal closure with towel clips (Fig. 4.1), followed by reopening and assessment of the amount of residual intra-abdominal hemorrhage. During this time, collective (i.e., surgery, anesthesia, blood bank) efforts are focused on normalizing temperature, acid–base status, and coagulopathy and critically reevaluating patient salvageability. One important exception to this practice is an isolated pelvic fracture with arterial hemorrhage, which warrants immediate angiographic intervention.
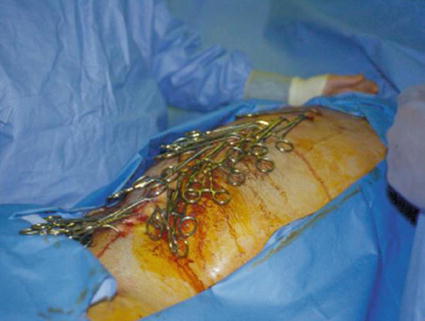

Fig. 4.1
Towel-clipped abdomen
After the brief period of towel clamp closure, the abdomen is examined for residual hemorrhage. Packs, except those successfully tamponading major hepatic venous injuries, are withdrawn sequentially to determine both efficacy and necessity (there are cases, although relatively infrequent, in which the packing can be completely removed and the fascia closed without incident). At this time, a search is undertaken for both residual mechanical bleeding and missed gastrointestinal injuries. In general, if more blood is present in the abdominal cavity than has been transfused during the period of towel clamping, surgical bleeding still exists and should be investigated. By contrast, bleeding from coagulopathy will only worsen if the operation is continued, and in this case, the patient should be closed temporarily and transported to the ICU, at which time damage control stage III begins. Although it is often uncomfortable to stop operating on a patient who is still bleeding, in the setting of profound shock and coagulopathy, this decision is usually lifesaving.
The final step in damage control stage II involves temporary wound closure. In the case of thoracic damage control via a lateral thoracotomy incision, the most rapid and simple technique involves stapled closure of skin only over tube thoracostomy drainage. In the case of a sternotomy, application of an adhesive, translucent dressing over the wound is sufficient. More options exist for laparotomy wound closure, ranging from towel clipping to achieve further tamponade to insertion of a translucent plastic dressing over the abdominal contents, over which drains may be placed and connected to a continuous suction to manage fluid efflux and monitor ongoing hemorrhage (Fig. 4.2). Disadvantages of the towel clip method include an increased likelihood of abdominal compartment syndrome (ACS) and inability to visualize the underlying bowel. Disadvantages of the latter method involve promotion of ongoing hemorrhage from the closed suction drains. None of these techniques has been proven superior to the others, and the overarching principle of rapid, controlled closure should not be overshadowed by the specifics of the dressing.
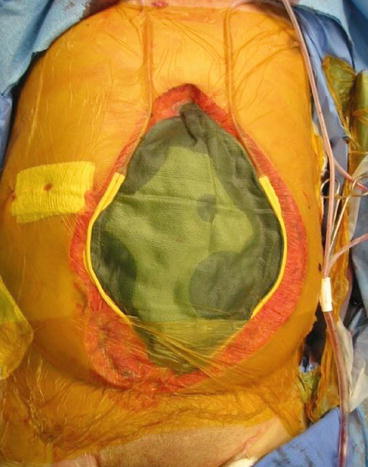

Fig. 4.2
Temporary abdominal closure with green towel, Jackson Pratt drains, and Betadine-impregnated adhesive dressing
Stage III, Physiologic Restoration in the ICU: The patient for whom a damage control approach has been selected usually arrives to the ICU in shock. As such, damage control stage III is centered on resuscitation. Hypothermia and coagulopathy are reversed aggressively. We favor goal-directed restoration of both enzymatic and platelet clotting function using serial TEG tracings. Although the optimal hemoglobin concentration during resuscitation from hemorrhagic shock remains unknown, a concentration of 8–10 g/dL is reasonable, and the decision to transfuse pRBCs should be based primarily upon clinical parameters (e.g., hemodynamic instability) and estimated ongoing blood loss from open wounds and drains. Many endpoints of resuscitation, such as serum lactate concentration, base deficit, and venous hemoglobin oxygen saturation, have been debated and are likely equivalent in terms of monitoring progress. Regardless of which marker is chosen, resuscitation should be guided by serial determinations and stop when normalization has occurred. Furthermore, utilization of multiple markers should be employed, such that the overall clinical picture is given preference over any one laboratory value. This strategy minimizes the possibility of misinterpreting isolated values and either terminating resuscitation prematurely or continuing with unnecessary and potentially deleterious volume expansion. A worsening base deficit in the otherwise resuscitated patient is usually due to a metabolic acidosis from large volume infusion of chloride-rich fluids (e.g., normal saline). Calculation of both the anion gap and serum chloride concentration will aid in this determination; a non-anion gap, hyperchloremic metabolic acidosis is characteristic. A worsening or persistently elevated lactate concentration in the otherwise resuscitated patient may be due to impaired hepatic clearance. Determination of a lactate/pyruvate ratio will elucidate this cause.
During damage control stage III, all organ systems should be supported, and no attempts at either liberation from mechanical ventilation or institution of enteral nutrition should be made until the patient is fully resuscitated. However, once resuscitated, institution of enteral nutrition (provided that the gastrointestinal tract is in continuity) is associated with improved subsequent fascial closure rates, morbidity, and mortality in open abdomen patients without associated intestinal injury and equivalent outcomes in those patients with associated intestinal injury [14]. It is thus the authors’ practice to institute enteral nutrition in the resuscitated damage control patient with an open abdomen.
Once the patient is warmed, and both enzymatic and platelet coagulopathy and metabolic derangements have been corrected, consideration is given to progression to damage control stage IV. It is important to recognize that, in contrast to the initial operation of damage control stage I, the reoperation of damage control stage IV is non-emergent. As such, the operation should occur after ensuring availability of blood products, personnel, and equipment. Abundant data now exists documenting the safety of maintaining intracorporeal laparotomy pads, indwelling vascular shunts, and gastrointestinal discontinuity for hours to days. Furthermore, time should be taken during damage control stage III to conduct a thorough evaluation for associated injuries that may have been overlooked heretofore.
Two instances in which consideration should be given to earlier return to the operation room warrant discussion. The first involves concern for ongoing surgical bleeding. Although differentiation from diffuse coagulopathy is often difficult, Morris et al. proposed indications for emergent reoperation during damage control phase III: for blunt trauma, normothermia with a rate of hemorrhage of >2 units pRBC per hour and, for penetrating trauma, either hypothermia with a hemorrhage rate of >15 units pRBC per hour or normothermia with a hemorrhage rate of >2 units pRBC per hour [15]. The second instance in which reoperation should be considered earlier is when the viability of bowel is in question, as is the case following ligation of a major mesenteric vein such as the portal or superior mesenteric. Furthermore, any patient who remains acidotic following correction of temperature, anemia, and coagulation status should be reexplored early with the specific concern of intestinal necrosis.
Stage IV, Return to the Operating Room for Definitive Procedures: At planned reoperation, intra-abdominal packing is removed, definitive vascular and intestinal tract repair is accomplished, a thorough exploration for missed injuries is undertaken, and fascial and skin closure may be performed provided that there is adequate laxity of the anterior abdominal wall tissues, and risk factors for the development of ACS are absent (discussed below). Packing should be removed carefully and after wetting to prevent dislodgement of formed clot. Persistent venous hemorrhage may necessitate repacking and a planned third operation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


