Primary Tumors of the Pleura
Malignant Mesotheliomas
Solitary Fibrous Tumors
Body Cavity Lymphoma
Pyothorax-Associated Lymphoma
MALIGNANT MESOTHELIOMAS
Malignant mesotheliomas are thought to arise from the mesothelial cells that line the pleural cavities. Individuals with a history of exposure to asbestos have a much greater risk of developing these neoplasms. Malignant mesothelioma with its dismal prognosis should be differentiated from the solitary fibrous tumor of the pleura, which has an excellent prognosis. A small percentage of mesotheliomas (<10%) arise in the peritoneal cavity (1), but only pleural mesotheliomas are discussed in this chapter.
Etiologic Factors
The occurrence of mesothelioma in many persons is related to previous exposure to asbestos. Asbestos is a fibrous silicate of various chemical types. The main types of asbestos are chrysotile and the amphiboles, which include crocidolite, amosite, tremolite, actinolite, and anthophyllite. Fibers with the greatest length-to-diameter ratio are the most carcinogenic (2,3). Exposure to the amphiboles is much more likely to induce mesothelioma than is exposure to chrysotile (3,4). In one study (5) of 118 mesotheliomas due to environmental exposure in South Africa, chrysotile was not implicated in any case. It has been estimated that the amphiboles are more than 750 times more potent that chrysotile in inducing mesothelioma (4). Indeed, in one model used to predict mesotheliomas in the future, exposure to chrysotile received no weight (6). Currently, chrysotile constitutes 99% of current global asbestos production. One of the possible reasons for the lower risk of malignancy from chrysotile exposure is that it is cleared from the lungs in a matter of hours to weeks, whereas the amphiboles are cleared only in a matter of decades (7). Crocidolite and amosite are the most carcinogenic amphiboles. Tremolite is a potent inducer of mesothelioma when the fibers have a high length-to-diameter ratio. No case of mesothelioma has been reported until now among Finnish miners exposed to anthophyllite asbestos, although there is a high incidence of pleural calcification because of this exposure.
Epidemiologic studies have implicated asbestos in the pathogenesis of malignant mesothelioma (8). In one study from Australia, 9 of 247 patients with the highest estimated cumulative exposure to asbestos had mesothelioma (8). It is thought that commercial amphiboles are responsible for most cases of mesothelioma observed in the United States (9). Occupations such as plumbing, pipe fitting, steam fitting, and mechanical engineering and industries such as ship- and boat-building have the highest risk of developing mesothelioma (10). In the United States, there have been 27 cases of pleural mesothelioma that developed in household contacts of asbestos workers (11). Most of the workers in these cases were shipyard workers or insulators, and the average latency after exposure before development of the tumor was more than 40 years (11).
Further evidence implicating asbestos as an etiologic agent in mesotheliomas comes from animal studies (see Chapter 4). Intrapleural injection of
any of the different types of asbestos results in the production of mesotheliomas in 8% to 66% of animals, depending upon the dose. Mesotheliomas can also be induced after the inhalation of various types of asbestos (12). These mesotheliomas are histologically identical to tumors in humans (13,14).
any of the different types of asbestos results in the production of mesotheliomas in 8% to 66% of animals, depending upon the dose. Mesotheliomas can also be induced after the inhalation of various types of asbestos (12). These mesotheliomas are histologically identical to tumors in humans (13,14).
The incidence of mesotheliomas following asbestos exposure increases linearly with the intensity of the exposure but exponentially (to the third or fourth power) with time from the first asbestos exposure. The risk of malignant mesothelioma can be estimated from the following mathematic equation:
R = K × F × Tp
where R is the risk of mesothelioma, K is a coefficient dependent on the fiber size and type (highest for crocidolite and lowest for chrysotile), F is the number of fibers per milliliter, T is the time after the first exposure, and p is the exponent that is thought to be between 3 and 4 (1,2). The most recent estimates for K for chrysotile and crocidolite were 0.013 and 1.0, respectively (2). The presence of pleural plaques or pleural thickening does not increase the risk of mesothelioma when the length and duration of exposure are taken into consideration (15).
At times, the asbestos exposure may not be obvious. In one report (16), five cases of mesothelioma developed in a Native American pueblo of approximately 2,000 persons. Epidemiologic investigation revealed that asbestos mats were used to insulate worktables against the intense heat of brazing torches and molten metal in the preparation of silver jewelry. In addition, the villagers scrubbed leather with cakes of asbestos to make their leggings and moccasins a brilliant white.
The mechanism by which asbestos fibers induce malignant changes is not known. The asbestos fiber appears to have two major sources of genotoxicity: generation of reactive oxygen species and mechanical effects such as interference with mitotic spindle formation and the segregation of chromosomes (17,18). Exposure to asbestos can also damage the cellular DNA, and if the cells with the damaged DNA either do not undergo apoptosis or undergo cell cycle arrest, a malignant transformation may occur. It should be noted that, however, so far no consistent abnormalities in oncogenes or suppressor genes have been found in human mesothelioma (1).
Mineral fibers other than asbestos can induce mesotheliomas. In one area of Turkey, approximately 1% of the population dies each year of malignant mesothelioma (19). A follow-up study in 2003 of 661 adult villagers alive in 1979 revealed that 119 (18%) had died of mesothelioma, and 19% of the mesothelioma deaths were in villagers less than 40 years old (20). Asbestos does not occur in the local soil or rocks, nor is it handled in the village. The atmosphere in the area does contain increased amounts of erionite, a mineral of the zeolite family. This mineral is a major contributor to the clouds in the area. This report demonstrates that the inhalation of airborne respirable fibers other than asbestos can be associated with the subsequent development of pleural mesotheliomas (19). In North Dakota, erionite has been found to contaminate gravel used to pave more than 300 miles of roads (21). It remains to be seen whether this contamination will lead to cases of mesothelioma in the future. When erionite fibers are administered intrapleurally to rats, they are two orders of magnitude more carcinogenic than crocidolite (22).
There are probably other factors related to the development of pleural mesothelioma. Antman et al. (23) reported that mesothelioma developed in proximity to a field of therapeutic radiation administered 10 to 31 years previously in four patients. As subsequent review of 9,342 patients who received radiotherapy for breast cancer revealed the three developed mesothelioma in the ipsilateral hemithorax (24). Roviaro et al. (25) reviewed 35 cases of pleural mesothelioma and found that 3 of the patients had calcified posttuberculous fibrothorax. There is no clear evidence of a familial tendency to develop mesothelioma (26). There is no definite evidence that smoking increases the risk of mesothelioma (1).
One factor that has received much attention in the last 20 years as a possible etiologic factor in the development of malignant mesothelioma is the simian virus 40 (SV40). It should be stated that the role of SV40 in mesothelioma is controversial and the possibility that technical factors can produce false-positive results suggestive of SV40 infection has been raised (27). Two more recent well-designed studies have shown that the polymerase chain reaction (PCR) primers used to detect SV40 in many studies were targeting sequences within the SV40 genome that are also present in commonly used laboratory plasmids, leading to the falsepositive detection of SV40 (28,29). A recent study using highly sensitive RT-PCR-based assays that are specific for SV40-encoded microRNAs found none in 94 malignant mesotheliomas (30). Moreover, agespecific trends in the pleural mesothelioma incidence rates are not consistent with an effect of exposure to SV40-contaminated poliovirus vaccine (31).
Incidence
The annual incidence of malignant mesotheliomas in the United States has been increasing over the last few decades and is thought to have peaked in 2004 with an annual incidence of approximately 2,300 (32,33). In comparison, the annual incidence of mesothelioma in western Europe in the year 2000 was 5,000 and is expected to peak around the year 2018 with an annual incidence of 9,000 (34). The difference in the incidence in the two locations is due to the fact that the maximal exposure to asbestos in Europe occurred around 1970, whereas the maximal exposure in the United States occurred from the 1930s to the 1960s (32). The incidence of mesothelioma in men is approximately six times that in women (10). The higher incidence in men is due, for the most part, to higher occupational exposure to asbestos. The seriousness of the problem with mesothelioma attributed to asbestos exposure is emphasized by the observation that, in Sweden, there are more deaths annually from mesothelioma due to asbestos exposure than to all fatal occupational accidents (35).
Pathologic Features
Malignant mesotheliomas in the earliest stages appear grossly as multiple white or gray granules, nodules, or flakes on normal or opaque parietal pleura (36). As the tumor progresses, the pleural surface becomes progressively thicker and nodular in appearance. The growing tumor extends in all directions to form a continuous layer encasing the lung and leading to contraction of the involved hemithorax. In advanced cases, the diaphragm, liver, pericardium, heart, contralateral pleura, and other mediastinal structures may be involved. At autopsy, hematogenously disseminated metastases are present in one third to one half of patients. In contrast to other sarcomas, however, the hematogenous metastases are usually clinically silent, and death generally results from complications arising from the primary lesion (1).
Microscopically, malignant mesotheliomas are characterized by marked structural variation within a single tumor or among different tumors with a similar gross appearance (37). Histologically, malignant mesotheliomas are classified as epithelial, sarcomatoid, or biphasic (38). Other classifications with more subtypes have also been developed (39). In a compilation of 819 cases from the literature, 50% were epithelial, 34% were biphasic, and 16% were sarcomatoid (40). The neoplastic cells of the epithelial form may show various epithelial arrangements such as papillary, tubular, tubulopapillary, cord-like, and sheet-like patterns. The epithelial cells may take various shapes but most commonly are cuboidal and uniform in size with vesicular nuclei. The sarcomatoid form resembles a spindle cell sarcoma in that the cells are spindle shaped with a parallel arrangement and have ovoid or elongated nuclei with well-developed nucleoli (41). The biphasic type has features of both the epithelial and sarcomatoid forms.
Clinical Manifestations
Two thirds of patients with malignant mesothelioma are between the ages of 40 and 70 years (42,43), and many have a history of exposure to asbestos for 20 or more years in the past. Most patients initially experience the insidious onset of chest pain or shortness of breath (44). Patients frequently have symptoms for several months before they see a physician (44). The chest pain is usually nonpleuritic and is frequently referred to the upper abdomen or shoulder because of diaphragmatic involvement. As the disease progresses, the patients lose weight and develop a dry, hacking cough and progressive dyspnea. Some patients have irregular episodes of low-grade fever (43). Physical examination may reveal clubbing. Examination of the chest reveals that the involved hemithorax is sometimes reduced in size and, at times, there is retraction of the intercostal spaces. In addition, there are the physical signs of a pleural effusion.
Radiographic Manifestations
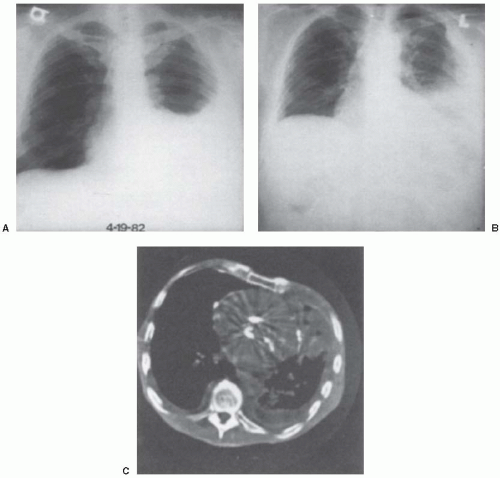
The chest radiograph (Fig. 11.1) reveals a pleural effusion in 75% to 90% of patients (45,46). This effusion is frequently large, occupying 50% or more of the hemithorax and obscuring the pleural tumor. In about one third of patients, pleural plaques are evident in the opposite hemithorax (47). With progression of the disease, the tumor encases the ipsilateral lung and thereby produces a mediastinal shift to the side of the effusion and results in a loculated pleural effusion. In the late stages of the disease, the chest radiograph may show mediastinal widening, enlargement of the cardiac shadow due to infiltration of the pericardium, and destruction of the ribs or soft tissue masses (48). At times, rounded atelectasis is present (see Chapter 27).
Because routine chest radiographs often underestimate the extent of the disease, chest computed tomography (CT) scans are invaluable in delineating the extent of the disease (45,46,47,48,49). Chest CT scans
should be obtained for all patients in whom a malignant mesothelioma is considered (Fig. 11.1C). With mesothelioma, the disease is unilateral in almost all cases (46). On CT scan, the pleura is thickened, with an irregular, often nodular internal margin that serves to distinguish this tumor from other types of pleural thickening. These changes are most pronounced at the base of the lung. The CT scan usually reveals marked thickening of the major fissure due to a combination of fibrosis, tumor, and associated fluid. The fissure may also appear nodular because of tumor infiltration (47). At times, pleural thickening is seen predominantly along the mediastinum. In such cases, the pulmonary margin is irregular, and separate nodules representing either metastases or lymph node infiltration may be seen in the juxtamediastinal tissue. In a patient with a malignant pleural effusion, features that suggest malignant mesothelioma rather than metastatic pleural disease are rind-like pleural involvement, mediastinal pleural involvement, and pleural thickness more than 1 cm (49).
should be obtained for all patients in whom a malignant mesothelioma is considered (Fig. 11.1C). With mesothelioma, the disease is unilateral in almost all cases (46). On CT scan, the pleura is thickened, with an irregular, often nodular internal margin that serves to distinguish this tumor from other types of pleural thickening. These changes are most pronounced at the base of the lung. The CT scan usually reveals marked thickening of the major fissure due to a combination of fibrosis, tumor, and associated fluid. The fissure may also appear nodular because of tumor infiltration (47). At times, pleural thickening is seen predominantly along the mediastinum. In such cases, the pulmonary margin is irregular, and separate nodules representing either metastases or lymph node infiltration may be seen in the juxtamediastinal tissue. In a patient with a malignant pleural effusion, features that suggest malignant mesothelioma rather than metastatic pleural disease are rind-like pleural involvement, mediastinal pleural involvement, and pleural thickness more than 1 cm (49).
The volume of the hemithorax with malignant mesothelioma is quite varied. If the patient has a pleural effusion with pleural thickening and decreased volume of the ipsilateral hemithorax, it is suggestive of mesothelioma. In one series, the volume
of the hemithorax was reduced in 42% of 50 cases of mesothelioma (45), whereas in another series, the volume of the hemithorax was reduced in 30% of 50 cases (46). It should be emphasized, however, that contralateral mediastinal shift is seen in approximately 15% to 25% of cases, and this is usually due to a large effusion (45,46).
of the hemithorax was reduced in 42% of 50 cases of mesothelioma (45), whereas in another series, the volume of the hemithorax was reduced in 30% of 50 cases (46). It should be emphasized, however, that contralateral mediastinal shift is seen in approximately 15% to 25% of cases, and this is usually due to a large effusion (45,46).
The CT scan is also useful in demonstrating disease beyond the pleura and is thereby quite useful in staging the disease. It often reveals intrapulmonary nodules that are not apparent on the standard chest radiograph (47). The CT scan may reveal chest wall invasion, diaphragmatic invasion, or extension of the tumor to the liver or retroperitoneal space. However, CT scans are not without problems with regard to mesothelioma; CT fails to identify chest wall and mediastinal invasion in some patients who undergo surgical resections (50). In addition, it is often very difficult to distinguish pleural disease alone from associated pericardial disease, and extensive pleural disease often envelops and obscures the nodal anatomy in the hilar and middle mediastinal nodal groups (45).
Magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG) positron emission tomography (PET) are other imaging modalities that are useful in the evaluation of patients with suspected malignant mesothelioma (51,52). MRI is superior to CT in determining the extent of a malignant mesothelioma particularly when the tumor invades local structures such as the ribs and the diaphragm (52). PET scans are useful in differentiating malignant pleural disease from benign pleural disease (51,53). In one series, 28 patients with pleural thickening, including 24 with malignant and 4 with benign disease, were subjected to FDG-PET scanning. The uptake of FDG was significantly higher in the malignant lesions than in the benign lesions. In addition, the FDG-PET images provided excellent delineation of the extent of the disease (51). However, it does not appear that metastatic adenocarcinoma can be differentiated from malignant mesothelioma with this imaging technique. In a paper reporting on PET scan results in 63 patients with mesothelioma, the authors concluded that the PET scan defines metastatic disease and delineates the extent of local disease as well (54). It has also been shown that the intensity of the FDG uptake on the PET scan is a significant factor in the prediction of patient survival (53).
Pleural Fluid
The pleural fluid with mesotheliomas is yellow in approximately 50% of patients and serosanguineous in the remainder. This fluid is exudative. In approximately one third of patients, the pleural fluid glucose level is below 50 mg/dL and the pleural fluid pH level is below 7.20 (55). Patients with a low pleural fluid pH or low pleural fluid glucose level tend to have a poorer prognosis (55). The pleural fluid is generally cellular and contains a mixture of normal mesothelial cells, differentiated and undifferentiated malignant mesothelial cells, and varying numbers of lymphocytes and polymorphonuclear leukocytes (56). Although measurement of the levels of tumor markers in pleural fluid is generally not recommended (see Chapter 7), malignant mesotheliomas tend to have high levels of CYFRA 21-1 and low levels of carcinoembryonic antigen (CEA) in comparison with metastatic adenocarcinoma (57).
It has been proposed that the demonstration of elevated levels of soluble mesothelin related protein (SMRP) are useful in the diagnosis of malignant mesotheliomas (58,59,60). Creaney et al (61) measured the SMRP levels in pleural fluids from 192 patients presenting to a respiratory clinic including 52 with malignant mesothelioma, 56 with nonmesotheliomatous malignancies and 84 benign effusions. The pleural effusions from the patients with mesothelioma had significantly higher concentrations of SMRP than did the other patients (61). However, the SMRP in patients with sarcomatoid mesothelioma did not differ significantly from nonmalignant effusions (61). Davies et al (60) measured pleural fluid SMRP in 24 patients with mesothelioma, 67 patients with pleural metastases and 75 patients with benign conditions. Using ROC curve analysis, pleural fluid SMRP had an AUC of 0.878 in its ability to differentiate between patients with mesothelioma and all other diagnoses at an optimal cutoff value of 20 nM (60). At this cutoff, the diagnostic sensitivity and specificity were 0.71 and 0.90, respectively (60). Again, the levels of SMRP were lower in patients that had sarcomatoid mesothelioma (60). Adenocarcinomas accounted for 12 of the 13 false positives. The above two studies demonstrate that pleural fluid SMRP measurement provide additional information to cytology. However, tissue confirmation of mesothelioma is indicated in most situations.
At times, the pleural fluid of patients with malignant mesothelioma is viscid, owing to the presence of large amounts of hyaluronate, which was previously called hyaluronic acid. Nurminen et al. (62) assessed the diagnostic utility of hyaluronate levels by assaying the levels in 1,039 samples of pleural fluids including 50 from mesothelioma. They found that with a cutoff of 75 mg/L for hyaluronate, the assay specificity
for malignant mesothelioma was 100% and the sensitivity was 56% (62). It should be noted that another study (63) demonstrated that the hyaluronate measurements were much less useful. It appears that the poor results in the latter study are probably attributable to procedural mistakes (64). The results by Nurminen et al. (62) were obtained by high-pressure liquid chromatography (HPLC), and this assay is not generally available in the United States.
for malignant mesothelioma was 100% and the sensitivity was 56% (62). It should be noted that another study (63) demonstrated that the hyaluronate measurements were much less useful. It appears that the poor results in the latter study are probably attributable to procedural mistakes (64). The results by Nurminen et al. (62) were obtained by high-pressure liquid chromatography (HPLC), and this assay is not generally available in the United States.
Diagnosis
The diagnosis of malignant mesothelioma should be considered in all patients with exudative pleural effusions. The suspicion of mesothelioma should be higher in middle-aged or older patients with persistent chest pain or shortness of breath, particularly if there is a history of asbestos exposure. The chest CT scan is frequently suggestive of the diagnosis. Although a diagnosis of malignancy can be established by cytologic smears or needle biopsies of the pleura, these procedures usually cannot distinguish between a metastatic adenocarcinoma and a mesothelioma. In one series (65), 80 patients with mesothelioma had pleural fluid cytology. In 20 patients (25%), cytologic examination of the pleural fluid established that the patient had malignant disease, but in none could the diagnosis of mesothelioma be established definitely with only cytology. In the remaining 60 patients, the diagnosis of malignancy could not be established (65).
There are, however, certain cytologic features that assist in making this differentiation. One report (66) compared the cytologic features of 44 cases of malignant mesothelioma and 46 cases of metastatic adenocarcinomas, and the authors concluded that the following five features separate malignant mesothelioma from adenocarcinoma with better than 95.4% accuracy. Mesotheliomas tend to have true papillary aggregation, multinucleation with atypia, and cell-to-cell apposition, whereas adenocarcinomas tend to have acinus-like structures and balloon-like vacuolation (66). In addition, immunohistochemical studies on cell blocks from pleural fluid are useful in distinguishing mesothelioma from adenocarcinoma (67,68,69) (see Chapter 7).
Blind needle biopsy of the pleura is usually not diagnostic of mesothelioma. In one report (65), the needle biopsy was diagnostic of mesothelioma in only 18 of 84 cases (21%). Also, there is poor concordance among different pathologists when the diagnosis of mesothelioma is based on specimens from needle biopsy (70). However, the diagnosis of mesothelioma can be established in more than 80% of patients with mesothelioma through a image-guided cuttingneedle biopsy (71,72,73) (see Chapter 28).
More invasive procedures are often necessary to provide a larger tissue sample so that a definitive diagnosis can be made. If the patient has skin deposits, these should be biopsied. However, usually the diagnosis must be made based on a thoracoscopy or open biopsy. Thoracoscopy establishes the diagnosis of mesothelioma in more than 90% of cases. When two series (74,75) were combined, the diagnosis was established in 51 of the 56 (90%) patients. These results were similar to those with thoracotomy and open biopsy (43). Thoracoscopy is therefore the procedure of choice because its diagnostic yield is similar to that of open thoracotomy, but the procedure is less invasive (43). It should be noted that the classification of the mesothelioma with thoracoscopy is wrong in about 15% of cases (76). Most cases of misclassification are diagnosed as an epithelial subtype at thoracoscopy and a biphasic subtype as the final diagnosis (76).
Malignant mesothelioma often infiltrates needle tracts, thoracotomy scars, and chest tube drainage sites after diagnostic or therapeutic procedures. In one study (73), the incidence of seeding was 4% for image-guided core-needle biopsy and 22% for thoracoscopic biopsy. The role of small amounts of irradiation after the procedure to prevent such seeding is controversial. Randomized studies have come to different conclusions (77). A recent review (77) on the subject concluded that prophylactic radiation was not justified.
Histologic examination of hematoxylin and eosin (H&E)-stained tissue section remains the primary method by which the diagnosis of malignant mesothelioma is established. Malignant mesothelioma can have many different histologic patterns (78). However, it is frequently difficult to distinguish malignant mesothelioma from metastatic adenocarcinomas on the H&E-stained slides (36,78). Currently, immunohistochemical procedures have gained widespread acceptance as valuable adjuncts in establishing the diagnosis of malignant mesothelioma (36,79). Some of the immunohistochemical markers are positive with adenocarcinomas, whereas others are positive with malignant mesotheliomas. When one wishes to differentiate metastatic adenocarcinoma from malignant mesothelioma, the tissue sections should be stained with a panel of immunohistochemical markers (see Chapter 7). Currently,
the best markers for mesothelioma appear to be calretinin, keratin 5/6, podoplanin, and WT1, whereas the best markers for metastatic adenocarcinomas are CEA, MOC-31, B72.3, Ber-EP4, BG-8, and TTF-1 (79) (see Chapter 7). The sarcomatous type of mesothelioma usually does not stain positively with calretinin (80). Moreover, it is important to realize that nonmalignant mesothelial cells also stain positive for cytokeratin 5/6 and calretinin (81). Indeed, one of the most difficult differentials is to distinguish malignant mesothelioma cells from benign reactive mesothelial hyperplasia (82). The most reliable criterion for determining that a mesothelial proliferation is malignant is the demonstration of invasion (82).
the best markers for mesothelioma appear to be calretinin, keratin 5/6, podoplanin, and WT1, whereas the best markers for metastatic adenocarcinomas are CEA, MOC-31, B72.3, Ber-EP4, BG-8, and TTF-1 (79) (see Chapter 7). The sarcomatous type of mesothelioma usually does not stain positively with calretinin (80). Moreover, it is important to realize that nonmalignant mesothelial cells also stain positive for cytokeratin 5/6 and calretinin (81). Indeed, one of the most difficult differentials is to distinguish malignant mesothelioma cells from benign reactive mesothelial hyperplasia (82). The most reliable criterion for determining that a mesothelial proliferation is malignant is the demonstration of invasion (82).
The diagnosis of malignant mesotheliomas is usually established by the combination of the histology and the immunohistochemical stains. However, when doubt exists, two tests that have been used for decades can at times still be useful. The periodic acid-Schiff (PAS) stain can still be used to distinguish mesotheliomas from adenocarcinomas. The presence of strongly positive vacuoles after diastase digestion effectively establishes the diagnosis of adenocarcinoma, although not all adenocarcinomas have this staining characteristic. In addition, most mesotheliomas contain large amounts of hyaluronate that stain positively with colloidal iron or alcian blue stains. To be unequivocally positive, absence or attenuation of blue staining after pretreatment of a serial section with bovine testicular hyaluronidase overnight is required (83).
The diagnosis of pleural mesothelioma is made accurately in most cases without resorting to electron microscopic (EM) examination (36). However, because EM still plays a decisive role in some cases with unusual morphology or anomalous histochemical or immunohistochemical reactions, a portion of the pleural specimen should be routinely fixed at the time of pleural biopsy for possible subsequent processing for EM if malignant mesothelioma is suspected (36). Epithelial mesotheliomas are characterized by the presence of tonofilaments, desmosomes, and microvilli. The appearance of the microvilli is important in distinguishing mesotheliomas from adenocarcinomas. In mesothelioma, the microvilli are numerous and are characteristically long and thin, whereas in adenocarcinoma they are typically much less frequent and are usually short and stubby (36,84). In one study (84), with scanning electron microscopy (SEM), the mean length-to-diameter ratio of the microvilli in mesotheliomas was 19.7:1 (range 13.7-23.5:1), whereas that for adenocarcinomas was 2.5:1 (range 1.3-4:1). SEM can be used when glutaraldehyde-fixed, plastic-embedded tissue is not available for transmission EM (84). A number of subcellular structures, such as mucin granules, myelinosomes, microvilli coated by a filamentous glycocalyx, and microvillous rootlets, may be observed in some adenocarcinomas. The presence of any of these features excludes mesothelioma (37).
Flow cytometry does not appear to be particularly useful in establishing the diagnosis of malignant mesothelioma. Burmer et al. (85) performed flow cytometry on 46 cases of malignant pleural mesothelioma and 31 nonmesothelioma malignancies of the pleural space. They reported that 65% of the mesotheliomas were diploid in DNA content, with intermediate-to-low proliferative rates. In contrast, 85% of the nonmesothelial malignant neoplasms were aneuploid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree