Fig. 11.1
The Kaplan-Meier curves show the main results of a randomized trial testing the use of a distal embolic protection device during PCI of saphenous vein aorto-coronary bypass grafts A 6.9 % absolute (42 % relative) reduction in the 30 days primary end point (a composite of death, myocardial infarction, emergency bypass, or target lesion revascularization) (p = 0.004) was observed in patients randomized to the distal embolic protection device GuardWire. Adapted from Baim et al. [4]
Proximal protection devices are an alternative to distal filters when the latter cannot be used. In the PROXIMAL trial these protection devices were non-inferior to distal devices in reducing the occurrence of peri-procedural AMI during PCI for obstructed saphenous vein grafts, also showing similar occurrence of major cardiac events [5]. In contrast, there is no evidence, at present, that the use of these device is efficacious during PCI of native coronary arteries [6].
11.1.2 Ischaemic Preconditioning
In one study ischemic preconditioning, obtained by two 90 s coronary balloon inflations, separated by 5 min reperfusion, prior to PCI was associated with a significant reduction of CK elevation [7] (Fig. 11.2). A similar beneficial effect was observed with remote ischemic preconditioning [8].
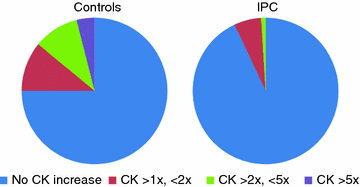

Fig. 11.2
Frequency of any CK elevation in patients undergoing PCI and randomized to ischemic preconditiong (IPC) or conventional PCI. CK elevation was seen in 7.1 % of patients undergoing PCI plus IPC and in 25 % of patients undergoing PCI without IPC (controls) (p < 0.005). Adapted from Laskey [7]
11.1.3 Pharmacological Prevention
11.1.3.1 Antiplatelet Drugs
As platelet activation is believed to play a relevant role in the pathophysiology of PCI-related myocardial infarction, antiplatelet and antithrombotic drugs have been proposed to prevent this complication or minimize myocardial damage.
Several studies have suggested that aspirin prevents peri-procedural Q-wave acute myocardial infarction [9, 10]. The dose suggested is 75−325 mg before PCI in patients who are already taking low-dose aspirin and 300−325 mg in patients not taking the drug [11].
Thienopyridines (clopidogrel, ticlopidine, prasugrel) are adenosine antagonists for P2y12 receptor [12]. In the ARMYDA-2 trial, a loading oral dose of clopidogrel, 600 mg, was associated with an approximately 50 % reduction of peri-procedural myocardial infarction as compared to a loading dose of 300 mg [13].
GPIIb/IIIa inhibitors also have been shown to reduce peri-procedural infarction in several clinical trials, a finding confirmed in a meta-analysis [14]. However, in the era of double platelet antiaggregation, GPIIb/IIIa inhibitors are recommended only in high risk patients with intracoronary thrombus [15].
11.1.3.2 Adenosine
Several pharmacological effects of adenosine might fight the occurrence of peri-procedural infarction during elective PCI (see also Chap. 10). Adenosine has been shown to decrease peri-procedural cardiac enzyme release in a small randomized study [16]. A more recent randomized placebo-controlled trial in non urgent PCI also showed reduction in peri-procedural AMI in patients randomized to receive intracoronary adenosine (50 mg) compared with placebo [17]. Larger studies are required, however, to establish the real clinical benefits of this approach.
11.1.3.3 Statins
Administration of atorvastatin was shown to reduce peri-procedural AMI during elective PCI compared to controls in the ARMYDA and in the NAPLES II trials [18, 19]. Also in patients with NSTE-ACS undergoing urgent PCI, both atorvastatin and rosuvastatin were shown to reduce peri-procedural AMI compared with controls [20, 21].
A meta-analysis [22] showed that statin administration prior to PCI almost halves the rate of peri-procedural myocardial infarction (Fig. 11.3).
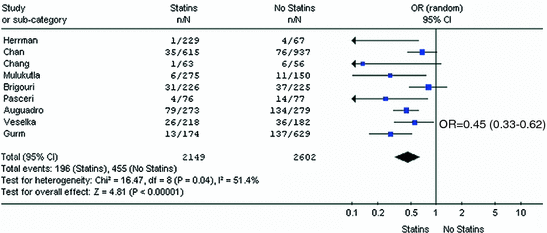

Fig. 11.3
The results of this meta-analysis show that statin administration prior to PCI almost halves the rate of peri-procedural myocardial infarction (OR 0.45, 95 %, CI 0.33–0.62, p < 0.01). Adapted from Merla et al. [22]
Of note, an atorvastatin load (120 mg) before PCI was shown to reduce post-procedural CK-MB and TnI elevation in patients with with NSTE-ACS who were on previous statin therapy in the ARMYDA-RECAPTURE trial [23], while this effect was not observed in patients with stable angina.
It is well accepted that the benefits deriving from statins during long-term follow-up are related to their lipid-lowering effect, whereas the potential clinical benefits related to their pleiotropic effects, including improvement of endothelial function and reduction of oxidative stress and of platelet adhesion have long been questioned [24]. However, the early benefits observed with statins within few hours of their administration in the setting of PCI are probably the most compelling evidence that pleiotropic effects likely play a relevant role in the clinical effects of these drugs.
11.1.3.4 Beta-Blockers
Intracoronary propranolol (15 µg/kg) before PCI was shown to reduce peri-procedural AMI during PCI, compared with controls [25], in particular when a GPIIb/IIIa inhibitor (eptifibatide) was added [26]. It has been suggested that the reduction in myocardial oxygen consumption might account for the beneficial effects of propranolol, although an increase in the endocardial/epicardial ratio of tissue perfusion in the ischemic area might also play a role [27].
11.1.3.5 Trimetazidine
Trimetazidine, a piperazine derivative, might prevent peri-procedural myocardial damage during PCI because of its cardioprotective properties [28].
A randomized, placebo-controlled trial showed that trimetazidine (loading dose 60 mg 30 min before recanalization) was associated with a significant reduction in post-procedural TnI levels [29]. The possible role of the drug in this context, however, needs further assessment in larger studies.
11.2 Prevention of CABG-Related CMD
Several pharmacological and non pharmacological approaches have been proposed to preserve coronary microcirculation and myocardial integrity in patients undergoing CABG.
11.2.1 Ischemic Conditioning
Ischemic preconditioning in the setting of CABG was the first ‘conditioning’ strategy applied in man [30]. In this study, patients undergoing CABG were randomized to preconditioning or the standard procedure. Preconditioning was induced by clamping and unclamping the aorta every 2 min, in order to cause brief episodes of global myocardial ischemia and reperfusion, before the sustained global myocardial ischemia induced by aortic cross-clamping during CABG surgery. Patients randomized to preconditioning had preserved ATP levels in ventricular biopsies and less peri-operative myocardial injury as evidenced by lower serum TnT concentrations.
A recent meta-analysis of 22 studies has been published, showing that preconditioning in this setting is associated with fewer ventricular arrhythmias and need for inotropic support (Fig. 11.4) [31]. However, due to the risk of arterial thrombo-embolism during clamping and unclamping of the aorta, it can be difficult to justify a large prospective study to definitely prove that preconditioning can improve clinical outcome.
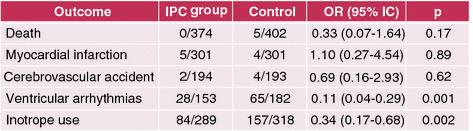

Fig. 11.4
The results of this meta-analysis show that ischemic preconditioning during cardiac surgery is associated with fewer ventricular arrhythmias and need for inotropic support. Adapted from Walsh et al. [31]
Besides before the sustained aortic cross-clamping, the ‘conditioning’ stimulus during CABG surgery can be applied at the end of the index global ischemic event, at the time of reperfusion (ischemic post-conditioning), when the patient comes off cardiac bypass and the heart is subjected to a period of global reperfusion. Beneficial effects have been reported with this strategy in one study [32]. However, whether post-conditioning can impact on clinical outcomes in patients undergoing CABG surgery remains to be determined.
Remote preconditioning also has been attempted [33]. In one study, patients undergoing elective CABG and valve surgery were randomized to remote preconditioning (three 5-min inflations/deflations of a blood pressure cuff placed on the upper arm) or control prior to surgery. Peri-operative myocardial injury (as measured by the 72 h area-under-the-curve of serum TnT concentrations) was reduced by 43 % in treated patients compared with controls. Subsequent studies, however, gave discordant results [34, 35], likely due to differences in remote preconditioning protocols, concomitant medications, patient selection and type of surgery. Thus a large randomized controlled clinical trial is warranted to investigate whether this intervention may offer cardioprotective effects in patients undergoing cardiac surgery.
11.2.2 Pharmacological Prevention
Several pharmacological cardioprotective strategies have been tested to protect the heart from the acute global ischemia–reperfusion injury during CABG. Drugs can be administered prior to aortic cross-clamping, added to the cardioplegic solution, or given at the time of aortic cross-clamp removal, or variably combining these different approaches.
11.2.2.1 Pharmacological Conditioning
Drugs believed to reproduce ischemic conditioning through their pharmacological effects have been tried in the setting of CABG.
Adenosine has been shown in several clinical studies to reduce peri-operative myocardial injury and improve cardiac indices when administered either as an intravenous therapy or when added to the cardioplegic solution [36–38]. However, the results of these studies are rather conflicting [39, 40].
Acadesine, which increases local availability of adenosine in ischemic tissues, showed initial benefits, but discordant effects were shown in subsequent trials [41–43].
Bradykinin has also been investigated in the setting of CABG surgery, but it only demonstrated a weak anti-inflammatory cardioprotective effect at the expense of significant hemodynamic instability [44, 45].
Diazoxide, a mitochondrial ATP-dependent potassium channel opener, administered during cardioplegia, improved functional recovery following CABG, but it did not reduce peri-operative myocardial injury as measured by CK-MB release [46, 47].
Volatile anesthetic agents have also been shown to induce conditioning in animal studies [48]. In patients undergoing CABG these drugs have been assessed in several studies [49, 50]. A meta-analysis including 2979 patients in 27 clinical trials showed that, compared with patients receiving intravenous anaesthesia, those who received volatile anesthetic agents had better cardiac function, less requirement for inotropic response, less peri-operative myocardial injury, as assessed by serum TnI levels, and a shorter duration of mechanical ventilation and hospital stay. However, no differences were observed in the incidence of infarction, intensive care unit stay and in-hospital mortality [49]. Other meta-analyses confirmed these data [50, 51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


