Chronic total occlusions (CTOs) represent a major challenge in percutaneous coronary revascularization. The recent advances in strategies and techniques and the development of dedicated equipment, such as microcatheters and wires, have resulted in considerably higher success rates. Although successful CTO recanalization is associated with significant clinical benefits, including improvement of angina, quality of life, reduced need for surgical revascularization, and improvement of left ventricular function, CTO procedures may require prolonged x-ray exposure and use of larger volumes of contrast compared with non-CTO procedures. Large volumes of contrast medium have been associated with increased rates of contrast-induced acute kidney injury (CI-AKI) and adverse short- and long-term outcomes. Application of specific measures and algorithms should be considered by all CTO operators to prevent CI-AKI.
Contrast-induced acute kidney injury (CI-AKI) is defined as the decrease of renal function that occurs in a narrow time window after administration of iodinated contrast agents and represents a major cause of adverse outcomes in patients who underwent percutaneous coronary intervention (PCI). CI-AKI is more prevalent in patients with pre-existing chronic kidney disease (CKD) and has been associated with high mortality rates, prolonged hospital stay, and increased health care costs. Contrast media volume has been shown to be an independent predictor for CI-AKI ; therefore, patients who underwent PCI for chronic total occlusions (CTOs) represent a high-risk group because of the use of large contrast doses and frequent need for repeat attempts.
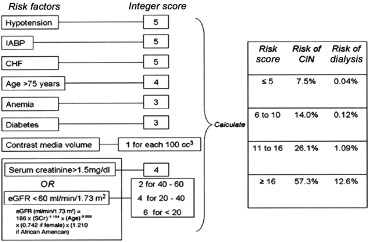
The most recently published Kidney Disease: Improving Global Outcomes definition of CI-AKI has been widely adopted by most of the national cardiovascular intervention societies and is listed in Table 1 . The incidence of CI-AKI in the most recently published registries and studies varies between 0.9% and 5.4% ( Table 2 ). No significant differences with regard to contrast volume have been shown between different subsets such as femoral versus radial access, in-stent restenosis versus de novo lesions, and patients with versus without previous coronary artery bypass grafting. The recent development of the “hybrid approach” to CTO crossing has resulted in a significant reduction in total fluoroscopy time and contrast utilization along with an improved technical success rate. The risk of CI-AKI in patients who underwent a coronary procedure may be estimated using a range of validated scores. The most commonly used scoring system, the Mehran score, is shown in Figure 1 . The incidence of CI-AKI in patients with pre-existing CKD who underwent standard PCI procedures has been reported as high as 55%. The correlation of contrast media volume during CTO PCI and the development of CI-AKI has been well documented in several studies. The estimated average amount of contrast load in CTO procedures is 350 ml compared with uncomplicated PCI procedures where it has been reported in the range of 150 to 200 ml. During CTO, patients who underwent PCI receiving ≥400 ml of contrast have an almost 2-fold higher incidence of CI-AKI compared with those receiving <400 ml of contrast. However, in the absence of coexistent CKD and diabetes mellitus, the incidence of CI-AKI remains low, even after high volumes of contrast media. Although the existing data are limited, no significant correlations between the CTO lesion characteristics and the development of CI-AKI have been shown. Lin et al showed that severe tortuosity of CTO lesions was an independent predictor of CI-AKI; however, none of the other established Multicenter CTO Registry of Japan score (J-CTO score) criteria for lesion complexity (calcification, length, ambiguous cap, previous unsuccessful attempt) reached statistical significance. In the same study, a Mehran score >11 was clearly correlated to the development of CI-AKI after CTO PCI. On the contrary, Aguiar-Souto et al showed that clinical parameters, procedural characteristics, target vessels, and Mehran scoring were not predictors for CI-AKI in CTO intervention. The risk factors for CI-AKI in CTO procedures are listed in Table 3 .
|
| Study type | Characteristics | Year | CTO lesions (n) | CI-AKI (%) | CI-AKI definition | Contrast (ml) | |
|---|---|---|---|---|---|---|---|
| Karmpaliotis et al | Registry | Retrograde only | 2012 | 462 | NR | NR | 345 ± 177 |
| Patel et al | Metanalysis | 2013 | 18941 | 3.8 | NR | NR | |
| Morino et al | Registry | 2010 | 528 | 1.2 | Increase in baseline SCr of at least 0.5 mg/dl in 48-72 hours | 293 (53-1097) ∗ | |
| El Sabbagh et al | Metanalysis | Retrograde only | 2014 | 3493 | 1.8 | NR | 350 ± 71 |
| Aguiar-Souto et al | Retrospective study | 2010 | 227 | 0.88-6.16 † | Increase in baseline SCr 25% or 0.5 mg/dl in 48 hours | 260 (200–350) ∗ | |
| Michael et al1 | Registry | 2013 | 1361 | NR | NR | 294 ± 158 | |
| Galassi et al | Registry | 2011 | 1983 | 0.9 | Increase in baseline SCr 25% or 0.5 mg/dl in 48 hours | 313 ± 184 | |
| Danzi et al | Registry | Multiple CTOs | 2013 | 249 | NR | NR | 400 (300-500) ∗ |
| Tsuchikane et al | Registry | Retrograde only | 2013 | 801 | NR | NR | 307 ± 137 |
| Lin et al | Retrospective study | 2014 | 516 | 5.4 | Increase in baseline SCr ≥25% in 48-72 hours | 296 ± 225 ‡ 277 ± 121 § | |
| Christopoulos et al | Registry | 2014 | 496 | NR | NR | 250 (180-360) ∗ |
† Depending on criteria used for diagnosis of CI-AKI.
| Patient-related | Procedure-related |
|---|---|
| Chronic kidney disease (eGFR <60 ml/min/1.73m 2 ) | High contrast media volume |
| Renal transplant | Multiple procedures |
| Older age (> 75 years old) | Lesion complexity (e.g. tortuosity of CTO segment) |
| Female sex | Intra-aortic balloon pump |
| Congestive heart failure | Hemodynamic instability |
| Left ventricular impairment | |
| Volume depletion | |
| Nephrotoxic medication (diuretics, non-steroidal anti-inflammatory drugs) | |
| Anemia | |
| Low serum albumin (< 35 g/L) |
Prevention of Contrast-Induced Nephropathy in CTO Procedures
Several preventive methods have been described in the literature; however, the data remain controversial for majority of them. So far, no strategies have been shown to be effective in preventing CI-AKI beyond thorough patient selection, minimizing the amount of contrast media and meticulous hydration of the patient. Preventive modalities can be classified in preprocedural methods, intraprocedural methods, and follow-up. A proposed algorithm for prevention of CI-AKI in CTO interventions is shown in Figure 2 .

The first step in prevention of CI-AKI in CTO procedures is the identification of patients at high risk. A thorough assessment of the risk factors and calculation of validated scores, such as the Mehran score, should be performed routinely for all patients in the CTO pre-assessment clinic. Nephrology consultation should be obtained before procedure for all patients at very high risk (estimated glomerular filtration rate [eGFR] <30 ml/min/1.73 m 2 ).
Hydration
Patients at high risk of CI-AKI should have a thorough assessment of their volume status and receive appropriate volume expansion before the procedure. Intravenous 0.9% sodium chloride has been shown to be more effective than 0.45% sodium chloride or oral hydration in prevention of CI-AKI. Although most of the trials have not directly addressed the ideal protocol, the most widely used approach is the administration of intravenous 0.9% sodium chloride at a rate of 1 ml/kg/hour for 24, beginning 12 hours before administration of the contrast medium, to achieve a urine output of >150 ml/hour. This approach seems to be superior to either bolus volume expansion during the procedure or removal of restrictions on oral fluid intake. Patients with moderate-to-severe left ventricular dysfunction should receive cautious hydration with isotonic 0.45% saline and close monitoring of urine output aiming to maintain a euvolemic state. The recently published Prevention of Contrast Renal Injury with Different Hydration Strategies (POSEIDON) trial showed that left ventricular end-diastolic pressure-guided volume expansion can effectively prevent CI-AKI in patients with renal impairment.
Calculation of maximum allowable contrast dose
In 1989, Cigarroa et al reported an empiric formula for calculating the maximal acceptable contrast dose (MACD):
MACD = 5 ml × weight ( kg ) / baseline serum creatinine ( mg / dl )
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


