Risk factors
OR
PAR (%)
Apo B/A1
3.2
49
Smoking
2.9
36
Psychological factors
2.7
33
Abdominal obesity
1.6
20
Hypertension
1.9
18
Diabetes mellitus
2.4
10
Fruits/vegetables
0.7
14
Physical activity
0.8
12
Alcohol
0.9
7
Over the last 15 years, we have learned important lessons on the relationship of risk factors to CVD, which are relevant to this question and pertinent to the polypill concept.
1.
For any given risk factor, the level of risk for death or an event is continuous along the entire range without any specific inflection point. This has been shown for systolic and diastolic blood pressure [12, 13], LDL cholesterol [10], number of cigarettes smoked (Fig. 29.1), or even WHR (waist to hip ratio) [10]. Conversely, meta-analysis of clinical trials demonstrated that a unit reduction of blood pressure [14] or LDL cholesterol [15] will lead to a proportionate reduction of risk of death irrespective of the original level of risk, although the absolute risk reduction will vary.
2.
Risk factors tend to cluster together. Thus, even if a person presents to the health system with one risk factor, e.g., hypertension, search for other common cardiovascular risk factors – tobacco use, abdominal obesity, diabetes mellitus, and hyperlipidemia – is required. In a population-based survey in India of 6,106 subjects, 77 % of those with hypertension, 68 % of those with prehypertension, and 46 % of those with normal blood pressure had additional cardiovascular risk factors [16]. Similarly, the Framingham Offspring Study showed that in those aged 18–74 years, less than 20 % of people had hypertension as an isolated risk factor [17].
3.
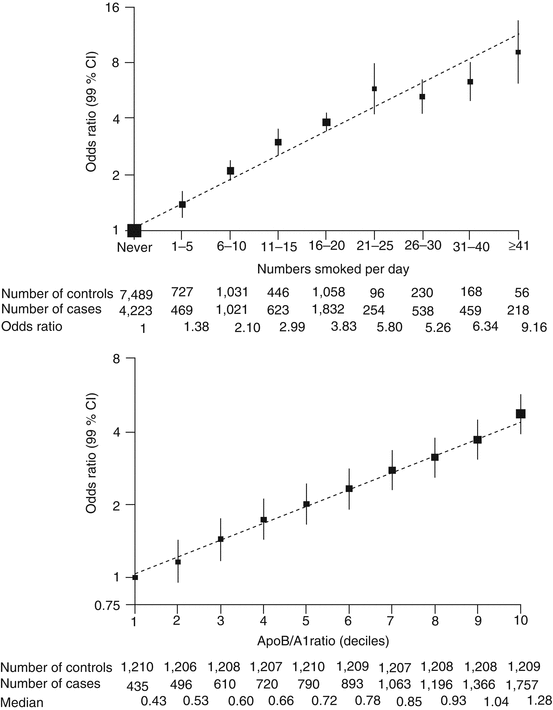
When risk factors occur together, the increase in risk is multiplicative rather than additive. In the INTERHEART study, while the odds ratio for acute myocardial infarction for current tobacco smoking, hypertension, diabetes mellitus, and hyperlipidemia (apoB/A1 ratio, highest vs lowest quintile) was 2.87, 1.91, 2.37, and 3.25, respectively, the combination of all of them, which is not uncommon, carried an odds ratio of 42.3 [10] (Fig. 29.2).
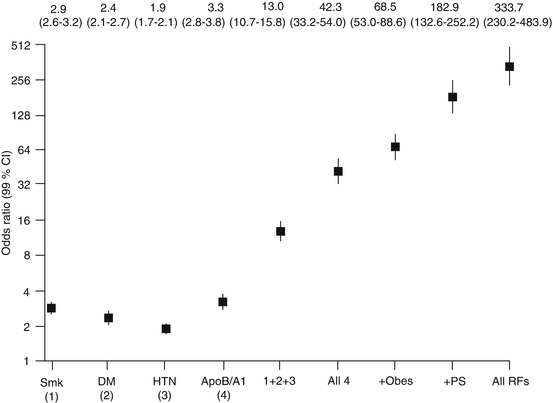

Fig. 29.2
Data from the INTERHEART study. While the odds ratios for acute myocardial infarction for current tobacco smoking, hypertension, diabetes mellitus, and hyperlipidemia (apoB/A1 ratio, highest vs lowest quintile) are 2.87, 1.91, 2.37, and 3.25, respectively, the combination of all of them, which is not uncommon, carries an odds ratio of 42.3 [10] (Adapted with permission from Elsevier Ref. [10])
4.
Given the distribution of common risk factors in the population, choosing a cutoff level at one end of the distribution tail as a target for intervention is likely to miss most people who will get the disease as most of them in absolute numbers will have risk factor levels below this cutoff in the “normal” range [18] (Fig. 29.3). Geoffrey Rose pointed out this paradox over 30 years ago [19].
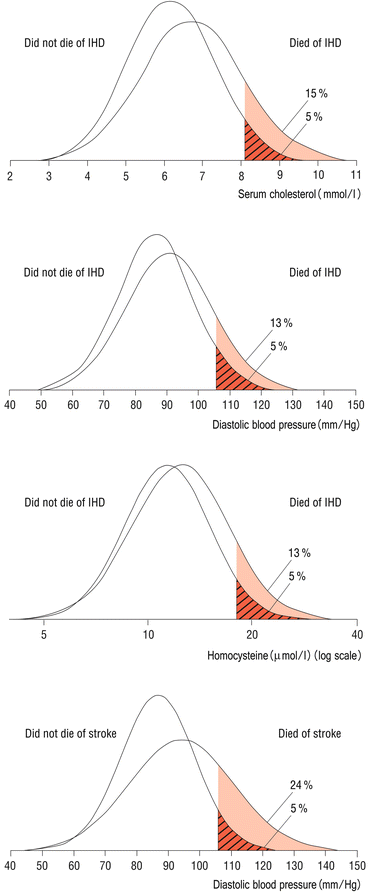

Fig. 29.3
Given the distribution of common risk factors in the population, choosing a cutoff level at one end of the distribution tail as a target for intervention is likely to miss most people who will get the disease as most of them in absolute numbers will have risk factor levels below this cutoff in the “normal” range [18] (Adapted with permission from BMJ Publishing Group Ltd Ref. [18])
The implications of this behavior of risk factors is that it is prudent to treat the person at risk rather than specific cutoff levels of risk factors (the latest NCEP guidelines for management of hyperlipidemia [20] have recommended this as well) and to address multiple risk factors simultaneously rather than piecemeal.
29.3 Levels of CVD Prevention
CVD prevention can be addressed at three levels – primordial, primary, and secondary.
Primordial prevention aims at preventing the development of risk factors in people and generally operates at the community or population level. Thus, in CVD prevention, the objective of primordial prevention would be prevention of tobacco use and reduction of mean population blood pressure, lipid levels, and obesity. At the population level, a small reduction of a risk factor, e.g., systolic blood pressure or LDL cholesterol, while having little impact on the individual could substantially reduce the burden of CVD at the country level. Primordial prevention has been described as one of the “best buys” in health promotion [21]. Primordial prevention largely requires action at the policy and political level through legislation and education through media.
An example of success in this strategy is the Polish experience [22] of using taxes and subsidies to reduce the consumption of butter, increase use of vegetable oils, and increase intake of fruits and vegetables with significant decrease in CVD mortality. Finland was one of the first countries to demonstrate the benefit of such prevention strategies [23].
Primordial prevention alone will not suffice, as it does not address individuals at high risk of disease who will need more intensive preventive measures. The current strategy for primary prevention involves identifying persons without CVD but at risk of getting it and treating them with both lifestyle modification and pharmacotherapy to reduce their risk factor levels to preset targets. Such a process involves screening asymptomatic individuals for level of risk (high risk, ≥20 % risk of event over 10 years; moderate risk, 10–19 % risk over 10 years; and low risk, ≤10 % risk over 10 years). This would involve measuring anthropometry (BMI and/or waist circumference), blood pressure, and usually lipids (although non-laboratory-based risk scoring systems are also available). National policies and guidelines should be available to recommend at which level of risk, intervention with lifestyle modification, and pharmacotherapy is to follow. Such levels may vary from country to country. Therapies are targeted to lower blood pressure and lipids and, possibly, inhibit platelet function with use of multiple medications. Subsequent follow-up of the subjects is required with repeat measurements of risk factors and adjustment of medication to achieve the required target risk factor levels. Such a strategy is not practical on a large scale, which is required to address the epidemic proportions of the problem. In addition, this strategy fails to take into account the continuous nature of risk factors by setting specific levels of risk factors at which to intervene.
Secondary prevention on the other hand requires less effort and cost to identify people requiring intervention since such people have already had a symptomatic coronary artery disease (CAD) or stroke event. They can be identified at the time of the index event or at subsequent follow-up visits. Such people are also likely to be more motivated both for follow-up and to adhere to pharmacotherapy and lifestyle modification. Yet even here, such adherence is less than desirable. The PURE (Prospective Urban Rural Epidemiology) study showed that in the community among 5,650 subjects with prior self-reported CAD events and 2,292 with stroke, overall only 25 % were taking antiplatelet medication, 17 % beta-blockers, 20 % ACEI/ARBs, and 15 % statins. Even in high-income countries, the percentages of those on medication were unsatisfactory (62, 40, 50, and 67 %, respectively), while it was abysmal in low-income countries (9, 10, 5, and 3 %, respectively) [24]. Further, the EUROSPIRE III survey found that among patients in high-income countries in Europe after a CAD event, 17 % still smoked tobacco, 35 % were obese, 56 % had suboptimal blood pressure control, and 25 % had elevated cholesterol [25].
In 2002, the WHO advised strategies designed for control of CVD at the population level. These called for:
1.
Education of policy makers and the public on the need for cardiovascular risk factor management.
2.
Shifting from a single risk factor approach to comprehensive risk management.
3.
Promotion of affordable approaches with rational resource allocation.
4.
Promotion of evidence-based lifestyle measures (smoking cessation, healthy diet, weight control, and increased physical activity).
5.
Use of cost-effective generic drugs. Subsequently, the World Health Assembly and the WHO accepted the prime importance of NCD and CVD control and defined national targets to achieve this goal. The four main targets are 25 % reduction in HTN, 30 % reduction in tobacco consumption, 30 % reduction in salt intake, and 10 % reduction in physical inactivity.
While lifestyle interventions are certainly of great importance and need to be maximally encouraged (Chap. 30) by governmental policies and media campaigns, they have generally not had a major sustained impact in reducing CVD events [26]. In people at moderate or greater risk and in those who have had a CVD event, pharmacotherapy is required. In situations where a large number of people need to be treated, the need for multiple tablets and the current requirement for screening and treating risk factors to preset targets may not succeed. It is in such a scenario that we have to consider the polypill concept.
29.4 The Polypill Concept
Broadly, the polypill concept is that in view of the fact that a unit of reduction in a risk factor will result in a constant proportional reduction in risk of events independent of the initial value of the risk factor, reducing a range of risk factors using a combination pill in all subjects with moderate or greater risk will reduce cardiovascular events significantly and be cost-effective. This hypothesis was proposed by Wald and Law in their seminal paper in 2003 [18]. They proposed a single pill containing medications to reduce BP, lipids, and homocysteine as well as platelet aggregation. They further proposed that in view of the fact that age is the strongest risk factor, instead of screening the population for risk factors to identify those at “moderate” risk, such a pill could be given to all people who have had a cardiovascular event (secondary prevention) as well as to all people over the age of 55 years (primary prevention) since this age itself will put them at moderate risk. As will be discussed below, using a series of meta-analyses, they hypothesized that such a strategy could lower CVD events significantly – CAD events by 88 % and strokes by 80 %. About one in three people would directly benefit each on average gaining 11–12 years of life free of heart attack or stroke. In those aged 55–64 years, this increase of disease-free life could be as much as 20 years. The antihypertensive composition of the pill proposed by them consisted of half doses of three drugs to reduce side effects. They also proposed the use of out-of-patent medications to reduce cost. The polypill concept is just that a hypothesis that including in a single pill different component to address multiple risk factors would be effective. If proven, the polypill hypothesis could bridge the gap between evidence and practice by improving access and adherence to medication with the possibility of large benefits at low cost.
29.4.1 Constituents of the Wald and Law Polypill
The polypill proposed by Wald and Law was to contain a statin (simvastatin 40 mg or atorvastatin 10 mg) to lower LDL cholesterol (Chap. 28), aspirin 75 mg as an antiplatelet agent (Chap. 23), folic acid 0.8 mg to lower homocysteine, and three blood pressure-lowering medications (Chaps. 36 and 38) at half dose (a beta-adrenergic blocker, e.g., atenolol 50 mg; an ACE inhibitor/ARB, e.g., ramipril 5 mg; and a diuretic, e.g., hydrochlorothiazide 12.5 mg). The reasons for including three blood pressure-lowering drugs in lower doses are:
1.
Each would act through a different pathway such that their effects on blood pressure lowering would be additive.
2.
Side effects of each of the components would be different and not additive. Lower doses would reduce side effects, as these are dose dependent.
3.
All the proposed constituents are no longer under patent and should serve to lower the cost of a polypill.
Wald and Law estimated that the statin in the pill (atorvastatin 10 mg or simvastatin 40 mg) would lower LDL cholesterol by over 1.8 mmol/L, which would be expected to lower CAD by 61 % and stroke by 17 %. The three antihypertensive components at the doses given should lower the diastolic BP by 11 mmHg resulting in reduction of CAD events by 32 % and stroke by 16 %. Aspirin should reduce IHD events by 32 % and stroke by 16 %. Using a series of meta-analyses, they calculated that such a pill could reduce CAD events by 88 % and strokes by 80 % (Table 29.2) [27, 28].
Table 29.2
Components of the polypill and estimated risk reductions achieved with individual components (Adapted with permission from BMJ Publishing group Ltd., Ref. [18])
Risk factor | Agent | Reduction in risk factor | % reduction in risk (95 % Cl) | |
|---|---|---|---|---|
IHD event | Stroke | |||
LDL cholesterol | Statin | 1.8 mmol/L | 61 (51–71) | 17 (9–25) |
Blood pressure | 3 classes of drugs at half standard dose | 11 mmHg diastolic | 46 (39–53) | 63 (55–70) |
Serum homocysteinea | Folic acid (0.8 mg/day) | 3 μmol/L | 16 (11–20) | 24 (15–33) |
Platelet function | Aspirin 75 mg/day | Not quantified | 32 (23–40) | 16 (7–25) |
Combined effect | All | – | 88 (84–91) | 80 (71–87) |
Since the publication of their paper, some of the assumptions made by Wald and Law have been questioned. Thus, clinical trials have shown the futility of folic acid supplements in reducing cardiovascular events [29]. This constituent is no longer considered for inclusion in the polypill. The value of aspirin in primary prevention of CVD is being questioned. The increased risk of bleeding due to aspirin may not be counterbalanced by the reduction of cardiovascular events in this population [30].
The polypill could have a number of potential benefits. It could decrease the cost of medication by, for example, reducing packaging and marketing costs as well as by using low-cost out-of-patent components. It could improve adherence by simplifying treatment regimens and make management by nonphysician health workers possible in select settings. These benefits could lead to greater efficiency of treatment, increased access, and equity and fewer medication errors on the part of the prescriber and the patient. On the other hand, there are potential risks. Formulation of a single pill with multiple components is not simple and may compromise bioavailability. The side effects of one drug may lead to discontinuation of all the components of the pill. While all the proposed components have been in use for secondary prevention and an effective combination pill could be used safely in this setting, the basic tenet that a simple one dose pill could be effective and safe in the reduction of cardiovascular mortality and morbidity in primary prevention remains unproven.
To test the polypill concept, the following steps would be required:
1.
Formulation of an effective multicomponent pill with pharmacokinetics and pharmacodynamics equivalent to those of the individual preparations
2.
Demonstration in clinical trials of the pill’s efficacy and safety in lowering risk factor levels in comparison to the individual components given separately
3.
Testing the basic premise of the hypothesis that treating subjects at risk for the primary prevention of CVD with a fixed drug combination without being guided by risk factor levels would actually lead to a reduction in cardiovascular events and be safe
29.4.1.1 Available Polypills
Pills with different components have been formulated. One of the first such is the Polycap (Cadila Pharmaceuticals, India). It is also a formulation that has and is undergoing the most extensive testing. It has been licensed in India for secondary prevention of CVD and has been marketed in that country since 2010. Its composition is aspirin 100 mg, simvastatin 20 mg, ramipril 5 mg, atenolol 50 mg, and hydrochlorothiazide 12.5 mg. A second version (full strength) is yet to be licensed but is being tested for primary prevention in the large ongoing event-driven TIPS 3 (The International Polycap Study 3) study. The latter pill contains simvastatin 40 mg, ramipril 10 mg, atenolol 100 mg, and hydrochlorothiazide 25 mg, but no aspirin. Other examples of cardiovascular polypills to our knowledge are shown in Table 29.3. Of these, the Cadila Polycap and the Ferrer polypill have been licensed for secondary prevention of CVD in India and South America, respectively.
Table 29.3
Polypill formulations, components, and sponsor
Polypill formulations | Sponsor | Components |
|---|---|---|
Polycap | Cadila Pharma, India | Simvastatin 20 mg, atenolol 50 mg, ramipril 5 mg, HCTZ* 12.5 mg |
Simvastatin 40 mg, atenolol 100 mg, ramipril 10 mg, HCTZ* 25 mg | ||
Red Heart Pill | Dr. Reddy’s Labs, India | Aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, atenolol 50 mg ± HCTZ 12.5 mg |
CardiaPill | CardioPharma, Inc., USA | Lisinopril, simvastatin, aspirin (strengths not available) |
PolyIran | Alborz Darou, Iran Tehran University < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|