Larry B. Goldstein
Prevention and Management of Ischemic Stroke
Each year, more than 795,000 Americans have strokes and more than 150,000 die, thus making stroke the country’s fourth leading cause of death.1 More than 25% of stroke survivors older than 65 years of age are institutionalized in a nursing home 6 months later.1 Stroke disproportionately affects minority populations. More than 60% of stroke-related deaths occur in women, and women are less than half as likely as men to be able to live independently after stroke.1 Although age is a major risk factor for stroke, more than a third of strokes occur in persons younger than 65 years of age.1 Risk factors for stroke overlap with those for coronary and peripheral artery disease, yet stroke can reflect a diverse set of pathophysiologic processes, and therapeutic interventions can confer levels of benefit and risk that differ from those for other forms of vascular disease.2 This discussion focuses on therapeutic interventions for prevention and treatment of stroke that are of particular relevance to cardiologists. The American Heart Association/American Stroke Association (AHA/ASA) has provided detailed, current evidence-based guidelines for prevention of a first stroke,2 prevention of recurrent stroke,3 and emergency management of patients with ischemic stroke.4
This chapter reviews various aspects of medical therapy for ischemic stroke. Readers are directed to AHA guidelines reviewing the use of carotid endarterectomy and angioplasty/stenting for primary and secondary prevention of stroke and to Chapter 60.5 COSS (Carotid Occlusion Surgery Study) found no benefit of extracranial-intracranial arterial bypass surgery over medical therapy in patients with symptomatic atherosclerotic internal carotid artery occlusion and hemodynamic cerebral ischemia (primary endpoint, stroke and death from surgery through 30 days and ipsilateral ischemic stroke within 2 years of randomization): 21.0% (95% confidence interval [CI], 12.8% to 29.2%) for the surgical group and 22.7% (95% CI, 13.9% to 31.6%) for the medical group (P = 0.78).6
Medical Therapy for Prevention of Stroke
Approximately 78% of strokes are first events, which makes primary prevention of paramount importance.1 Prevention of recurrent events is also critical. Depending on age and race or ethnicity, between approximately 6% and 25% of survivors will have a second stroke within 5 years.1 The risk for ischemic stroke after a transient ischemic attack (TIA, a frequently misdiagnosed condition, is defined as “a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction”) is as high as 17% over a 90-day period (with the highest risk in the first week).7 The ABCD2 score can be helpful in assessing the short-term risk for stroke in patients with TIA (Table 59-1).8 The risk for stroke within 2 days is low (1%) in those with a score of 0 to 3, moderate (4%) in those with a score of 4 to 5, and high (8%) in those with a score of 6 to 7.8 Several studies suggest that patients with a low ABCD2 score can be evaluated rapidly and managed in an outpatient, specialized clinic or a similar unit, but even those at apparent low risk may have a condition requiring urgent treatment.9 The risks and benefits of therapeutic interventions differ for primary and secondary prevention of stroke.
TABLE 59-1
Short-term Risk for Stroke after Transient Ischemic Attack: ABCD2 Score
| FACTOR | POINTS |
| Age >60 yr | 1 |
| Blood pressure >140/90 mm Hg | 1 |
| Clinical features | |
| Speech deficit, no weakness | 1 |
| Unilateral weakness | 2 |
| Diabetes | 1 |
| Duration | |
| 10-59 min | 1 |
| >60 min | 2 |
Platelet Antiaggregants
Primary Prevention
The use of platelet antiaggregants for primary prevention of stroke depends on the patient’s global risk for cardiovascular events and stroke. No evidence has shown that platelet antiaggregants reduce the risk for stroke in persons at low risk.2
The benefit of aspirin for primary cardiovascular prophylaxis outweighs its associated risk for bleeding complications in persons with a 10-year risk for coronary heart events of 6% to 10%, but there is no evidence of a reduction in risk for stroke even in these patients (predominately men), and aspirin is not recommended for this purpose.8 Although the Women’s Health Study found no reduction in its prespecified primary endpoint (nonfatal myocardial infarction [MI], nonfatal stroke, or cardiovascular death) with aspirin (100 mg on alternate days), there was a 17% reduction in the risk for stroke, albeit with an increase in the risk for bleeding.10 This benefit occurred primarily in women at increased risk for stroke because of the presence of other factors (e.g., hypertension, diabetes). Thus aspirin may be considered in women whose risk for stroke outweighs its associated bleeding risk.8 There is no evidence of benefit in reducing the risk for a first stroke with any other platelet antiaggregant.
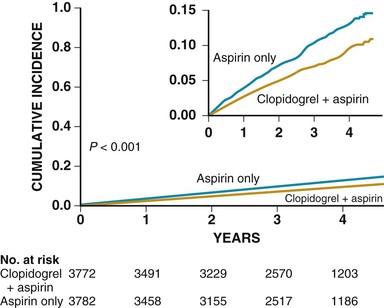
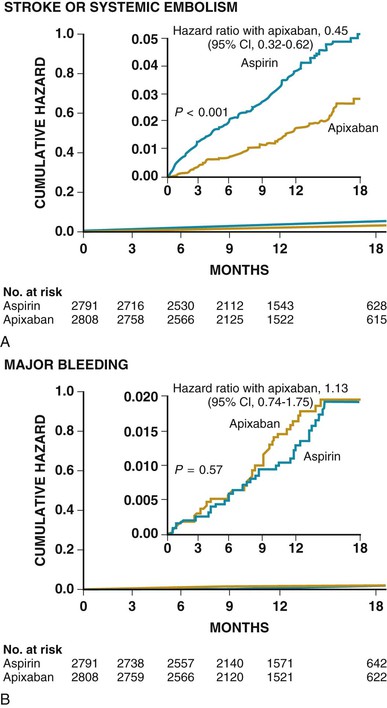
Anticoagulation is generally recommended for prevention of stroke in patients with atrial fibrillation who have a high risk for systemic embolization.3 In addition to warfarin, new agents have become available for prevention of a first and recurrent stroke in patients with nonvalvular atrial fibrillation (see later and Chapters 38 and 82).3 Aspirin plus clopidogrel may decrease the risk for major vascular events (MVEs, predominately stroke) and stroke (Fig. 59-1) in comparison to aspirin alone in patients with atrial fibrillation who cannot take anticoagulants, but the combination is associated with an increased risk for major bleeding complications such that no overall net benefit was achieved (MVEs decreased 0.8% per year, major hemorrhages increased 0.7% per year; relative risk [RR], 0.97; 95% CI, 0.89 to 1.06; P = 0.54).11 Apixaban may be superior to aspirin in preventing stroke or systemic embolization, and has a similar rate of major bleeding (Fig. 59-2).3,12
Secondary Prevention
Aspirin (lowest efficacious dose in comparison to placebo, 50 mg/day) lowers the risk for recurrent stroke by approximately 15% in persons with noncardioembolic ischemic stroke.7 Sustained-release dipyridamole (200 mg twice daily) is as efficacious as aspirin in reducing the risk for recurrent stroke, with a further reduction (≈37%) when the two drugs are combined.7,13 Aspirin plus sustained-release dipyridamole is available in the United States in a fixed-dose combination (25 mg aspirin plus 200 mg dipyridamole) that is given twice daily. Cardiologists are often concerned that dipyridamole might increase the risk for cardiac ischemia, but clinical trials have not substantiated this reservation. There is also concern that the total dose of aspirin (50 mg/day), although efficacious for secondary stroke prophylaxis, is below the dose shown to be effective for cardiac prophylaxis. To address this potential limitation, a small additional dose of aspirin can be added (e.g., 81 mg/day) to the fixed combination of aspirin and dipyridamole.13
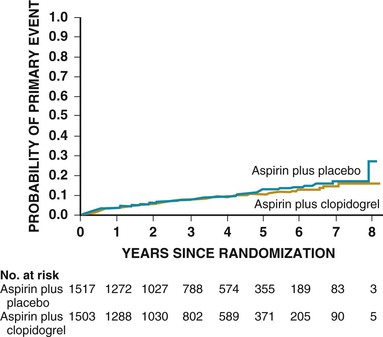
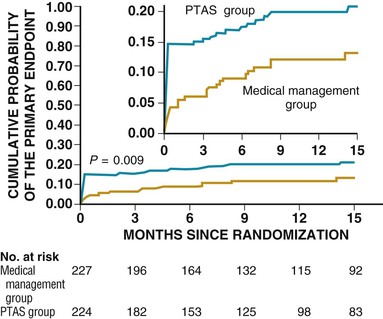
Clopidogrel monotherapy given to patients with a history of MI, stroke, or symptomatic peripheral arterial disease reduces the combined risk for MI, stroke, or vascular death by 8.7% as compared with aspirin.7 Although based on a potentially underpowered subgroup analysis, there is no evidence of a significant reduction in stroke in those with previous stroke. 7 No reduction in a composite endpoint of MI, stroke, or cardiovascular death in patients with cardiovascular disease (including stroke) or multiple risk factors was found with aspirin plus clopidogrel versus aspirin alone.14 When tested directly in patients with stroke, the combination of aspirin and clopidogrel was associated with an increase in bleeding complications without a reduction in ischemic stroke.15 SPS3 (Stroke Prevention Study 3) similarly found a higher risk for hemorrhage with no reduction in ischemic events after lacunar stroke in those treated with the combination versus aspirin alone (Fig. 59-3).16 Aspirin and clopidogrel should not be used in combination for stroke prophylaxis in patients at high risk or in patients with recent stroke.7
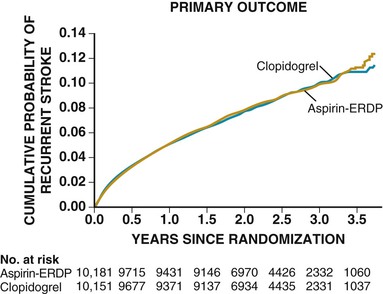
A direct comparison found that aspirin plus dipyridamole was comparable to clopidogrel monotherapy for secondary stroke prevention in patients with noncardioembolic stroke (Fig. 59-4).17 Aspirin, aspirin plus sustained-release dipyridamole, and clopidogrel are reasonable options for secondary prevention in these types of patients.7
Anticoagulation
Primary Prevention
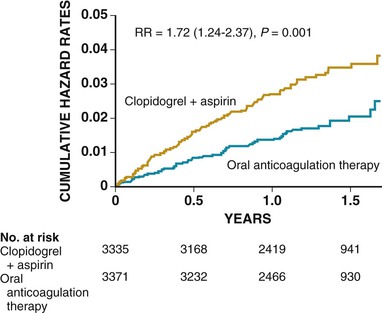
The use of long-term anticoagulation to reduce the risk for a first cardiogenic embolism in patients at increased risk because of conditions such as mechanical heart valves, atrial fibrillation, and cardiomyopathy is addressed in Chapters 25, 38, 63, and 82. The combination of aspirin and clopidogrel is inferior to warfarin for stroke prevention in patients with atrial fibrillation (Fig. 59-5).18
Secondary Prevention
Evidence supporting the use of anticoagulation for prevention of recurrent stroke in patients without atrial fibrillation or other high-risk cardiogenic sources is uncertain, or the evidence suggests that the benefit does not outweigh the risk for warfarin-associated bleeding complications.
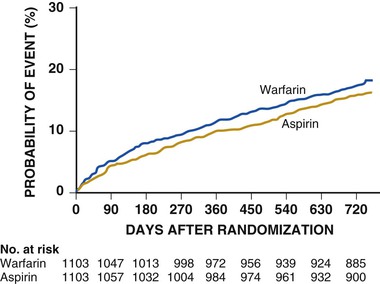
For patients with noncardioembolic stroke, WARSS (Warfarin-Aspirin Recurrent Stroke Study) directly compared warfarin (international normalized ratio [INR] of 2 to 3) and aspirin (325 mg/day).19 A nonsignificant advantage was associated with aspirin treatment (17.8% rate of recurrent stroke or death with warfarin versus 16.0% with aspirin, P = 0.25) (Fig. 59-6). The use of antiplatelet agents rather than oral anticoagulation is recommended to reduce the risk for recurrent stroke and other cardiovascular events in patients with a noncardioembolic stroke or TIA.7

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree