Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome, often occurring in young women. The utility of comprehensive imaging and clinical significance of detected vascular abnormalities have yet to be determined. We hypothesized that extracoronary vascular abnormalities (EVAs) are common in SCAD and aimed to study the prevalence and distribution of these findings. We enrolled 115 patients with confirmed SCAD who were evaluated at the Mayo Clinic SCAD Clinic from February 2010 to May 2014 and prospectively underwent comprehensive computed tomography angiography imaging of the neck, chest, abdomen, and pelvis (SCAD computed tomography angiography protocol, n = 95) or had retrospective review of outside studies (n = 20) including head imaging (n = 40). Follow-up was determined by last clinical visit or study correspondence and included review of recurrent SCAD or myocardial infarction, congestive heart failure, and death. We reported EVAs in 66% of patients with SCAD, most frequently in the abdomen (36%), pelvis (28%), and neck (27%). Only 1 patient had EVA in the chest (aortic dissection and Marfan’s). Fibromuscular dysplasia (FMD) (exclusively multifocal) was the most common type of EVA (45%). Vascular abnormalities in those with head imaging included intracranial aneurysms (n = 9) and FMD (n = 3). There were no deaths at median follow-up of 21 months (Q1 to Q3 7.7 to 55). The presence of FMD was not associated with SCAD recurrence (relative risk [RR] 1.2; confidence interval [95% CI] 0.60, 2.5), congestive heart failure (RR 0.66; 95% CI 0.20, 2.3), or myocardial infarction (RR 1.34; 95% CI 0.69, 2.6). In conclusion, EVAs including FMD, dissections, aneurysms, and dilation are common in patients with SCAD and occur in a wide anatomic distribution. The presence of EVAs and/or FMD did not correlate with the risk of subsequent clinical events, but future studies with increased power and longer follow-up will be important to further assess the role of EVAs in patients with SCAD.
Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome most frequently affecting young and otherwise healthy women. Although SCAD has been associated with a number of potentially exacerbating factors including the peripartum status, connective tissue diseases, extreme exercise, and stress, it is most notably associated with fibromuscular dysplasia (FMD). FMD is a nonatherosclerotic, noninflammatory vascular abnormality typically involving the small-medium muscular arteries without clear underlying cause and also commonly affects young women. The association of FMD with SCAD prompts concern for an underlying systemic vasculopathy. The utility of comprehensive imaging and the clinical significance of detected extracoronary vascular abnormalities (EVAs) have yet to be determined as there have been no studies to date using imaging to extensively evaluate the distribution of EVAs and FMD in a large SCAD population. Therefore, we comprehensively imaged patients with SCAD for detection of EVAs and FMD to (1) define the prevalence of EVAs and FMD in our cohort, (2) describe the distribution of vascular abnormalities, and (3) determine the utility of comprehensive vascular imaging and its potential association with clinical findings and outcomes.
Methods
We analyzed patients evaluated at the Mayo Clinic SCAD Clinic from February 2010 to May 2014 who had been previously diagnosed with SCAD, defined as acute coronary syndrome with coronary angiography and/or intravascular imaging demonstrating diagnostic features of SCAD (intimal dissection and intramural hematoma) as confirmed by at least 2 interventional cardiologists with extensive experience in SCAD diagnosis. Evaluation for EVAs was also performed on femoral/radial angiography if recorded at the time of cardiac catheterization (n = 30).
Patients with SCAD prospectively underwent computed tomography angiography (CTA) imaging protocol of the neck, chest, abdomen, and pelvis developed specifically for them (SCAD computed tomography angiography protocol [SCTAPr], n = 95). Patients with SCAD with extracoronary imaging including body CTAs and/or magnetic resonance angiographies (MRAs) performed elsewhere were also included (n = 20). Head CTA or MRA was reviewed when available (n = 40). All head and neck images were interpreted by dedicated neuroradiologists, and all chest, abdomen, and pelvis images were interpreted by dedicated vascular radiologists. In addition, a senior vascular radiologist with >20 years of experience (TJV) reviewed all neck, chest, abdomen, and pelvis images for consistency of acquisitions, accuracy of findings, and clarity of interpretation. Baseline characteristics and clinical course were abstracted from the information provided during the clinical visit including physical examination, medical records, and questionnaires. The patients continue to be prospectively followed through clinical or research correspondence. The Mayo Foundation Institutional Review Board approved this study, and all patients had provided previous authorization for the use of their medical information for research purposes.
The SCTAPr imaging was obtained on a 128-slice single-source CT scanner (Definition AS; Siemens Healthcare, Forchheim, Germany) or a 128-slice dual-CT scanner operated in single-source mode (Definition Flash; Siemens Healthcare) with an injection of 200 cc of the low-osmolar contrast agent, iohexol (Omnipaque 350), at 5 cc/s followed by 25 cc of normal saline as described previously. After instruction and practice of arm positioning maneuvers, each subject underwent a dedicated neck CTA followed immediately by CTA of the chest, abdomen, and pelvis in a single acquisition. Images were sent to a 3-dimensional postprocessing laboratory for volume-rendered imaging of the neck, chest abdomen, and pelvis. Average volume CT dose index was 10.5 mGy for the chest, abdomen, and pelvis and 24.3 mGy for the neck portion.
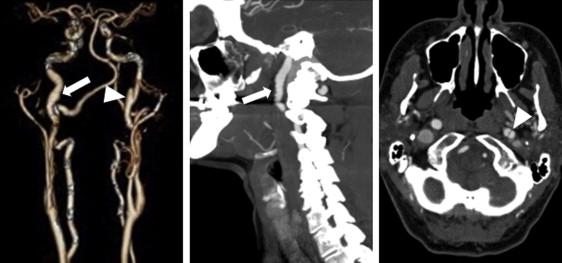
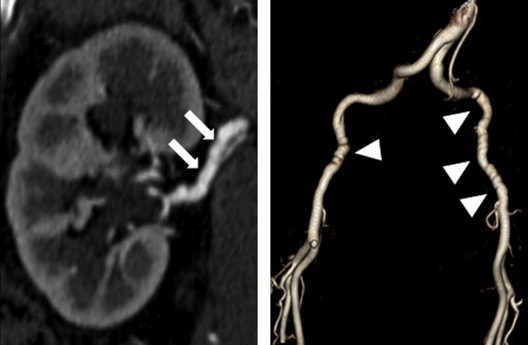
FMD was defined according to the American Heart Association classification as (1) multifocal—the presence of sequential focal narrowing separated by focal dilations such as depicted in classic “string of beads” appearance ( Figures 1 and 2 ) and (2) unifocal—segments of tubular stenosis. “Aneurysm” was defined as a >50% enlargement in the diameter of an artery compared with an adjacent normal arterial segment. “Dilation” was defined as the presence of a visually dilated artery with a diameter that was increased but <50% larger than an adjacent normal arterial segment. “Dissection” was defined as the presence of a dual lumen vessel ( Figure 1 ). “Undulation/tortuosity” was defined as an apparent deviation in arterial curvature ( Figure 3 ).
Statistical analysis was performed with JMP, version 10.0.0 (SAS Institute, Inc, Cary, North Carolina). Continuous data were summarized as a mean (SD), and comparisons were performed with the Student’s t test. Frequencies and percentages were used to express discrete variables, and comparisons were performed by a Fisher’s exact test. Risk ratios for clinical outcomes were estimated using Cox proportional hazards models. A 2-sided p value <0.05 was considered statistically significant.
Results
We evaluated 115 patients with a history of SCAD at our institution with either comprehensive SCTAPr imaging (n = 95) or limited imaging (n = 20). A substantial majority were women, and mean age at the time of SCAD event was 43 ± 9 years ( Table 1 ). The technical success rate of the SCTAPr was 97%. The early technical failure of neck imaging in 3 patients resolved with enhanced technologist training and patient instruction.
| Baseline Characteristics | All Patients (n=115) | SCTAPr (n=95) | Limited Screen (n=20) |
|---|---|---|---|
| Age at SCAD diagnosis, mean ± SD, range (years) | 43 ± 9, 28-72 | 44 ± 9, 28-72 | 45 ± 8, 31-57 |
| Female | 109 (95%) | 92 (97%) | 17 (85%) |
| Caucasian | 112 (97%) | 92 (97%) | 20 (100%) |
| South Asian | 2 (2%) | 2 (2%) | 0 |
| Hispanic | 1 (0.9%) | 1 (1%) | 0 |
| Body Mass Index, mean ± SD, range (kg/m 2 ) | 26 ± 6, 17-46 | 26 ± 6, 17-46 | 26 ± 6, 19-45 |
| Hypertension | 32 (28%) | 26 (27%) | 6 (30%) |
| Hyperlipidemia | 36 (31%) | 30 (32%) | 6 (30%) |
| Active Tobacco Use | 10 (9%) | 6 (6%) | 4 (20%) |
| Prior Tobacco Use | 26 (23%) | 20 (21%) | 6 (30%) |
| Diabetes Mellitus | 2 (2%) | 2 (2%) | 0 |
| Migraines | 53 (46%) | 41 (43%) | 12 (60%) |
| Marfan Syndrome | 1 (0.9%) | 0 | 1 (5%) |
| Mixed Connective Tissue Disease | 1 (0.9%) | 1 (1%) | 0 |
| Family history of: | |||
| Early myocardial infarction in 1 ◦ relative | 1 (0.9%) | 1 (1%) | 0 |
| Intracranial aneurysm in 1 ◦ relative | 4 (3%) | 3 (3%) | 1(5%) |
| Sudden death in 1 ◦ relative | 2 (2%) | 2 (2%) | 0 |
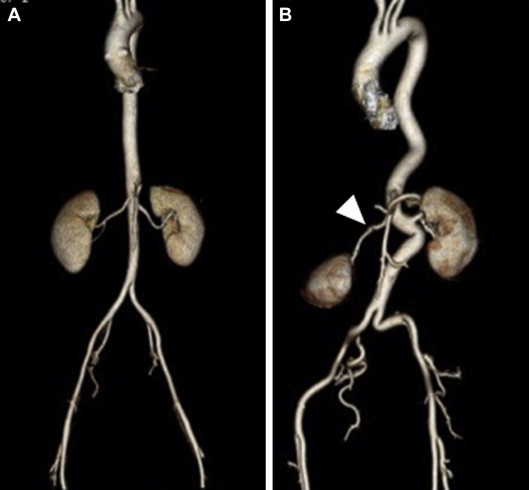
Of 115 patients who underwent any form of vascular imaging, EVAs were present in 66% and was most frequent in the abdomen, followed by the pelvis and neck ( Table 2 ). Of those with EVAs, 74% had >1 abnormal vessel and 29% had abnormalities in >1 body territory. EVA in the chest was identified in 1 patient who had known Marfan’s disease and chronic aortic dissection. FMD (exclusively multifocal) was the most common EVA, occurring most frequently in the renal, carotid, and iliac arteries. Patients who underwent limited screening were less likely to be diagnosed with EVAs and FMD compared with those who underwent the comprehensive SCTAPr (40% vs 72%, p = 0.0096; 15% vs 52%, p = 0.0029, respectively). There was no association between the presence of intracranial aneurysms (n = 9) and concurrent EVAs elsewhere (1 of 9 without EVAs vs 8 of 9 with EVAs, p = 0.4) or FMD (4 of 9 without FMD vs 5 of 9 with FMD, p ≥0.99). Migraines were reported by nearly one-half of all patients, and 8 of 20 patients with migraine with intracerebral imaging had abnormalities. No venous vascular abnormalities were identified. In our earlier series, secondary review of SCTAPr clinical studies (TJV) noted the occasional lack of reporting by the interpreting radiologists of subtle imaging findings, such as mild FMD, dilation, or tortuosity (5 of 39 patients) ( Figure 4 ). Familiarity with these EVAs in the SCAD population increased subsequent reporting by the interpreting radiologists.
| Distribution | All Patients (n=115) | SCTAPr (n=95) | Limited Screen (n=20) |
|---|---|---|---|
| Total EVA | 76 (66%) | 68 (72%) | 8 (40%) |
| Total FMD | 52 (45%) | 49 (52%) | 3 (15%) |
| EVA >1 territory | 33 (29%) | 32 (34%) | 1 (5%) |
| No. arterial beds involved | 1.7 ± 2 (1-8) | 1.9 ± 2 (1-8) | 0.5 ± 1 (1-5) |
| Cervical | 31 (27%) | 29 (31%) | 2 (10%) |
| Carotid FMD | 25 (22%) | 23 (24%) | 2 (10%) |
| Carotid dissection | 5 (4%) | 5(5%) | 0 |
| Carotid aneurysm | 3 (3%) | 3 (3%) | 0 |
| Carotid dilatation | 2 (2%) | 2 (2%) | 0 |
| Carotid “looping” | 3 (3%) | 3 (3%) | 0 |
| Vertebral FMD | 7 (6%) | 6 (6%) | 1 (5%) |
| Vertebral dilatation | 1 (0.9%) | 1 (1%) | 0 |
| Vertebral tortuosity | 1 (0.9%) | 1 (1%) | 0 |
| Thorax | 1 (0.9%) | 0 | 1 (5%) |
| Aortic dissection | 1 (0.9%) | 0 | 1 (5%) |
| Visceral | 41 (36%) | 38 (40%) | 3 (15%) |
| Renal FMD | 33 (29%) | 32 (34%) | 1 (5%) |
| Renal aneurysm | 2 (2%) | 2 (2%) | 0 |
| Celiac dilatation | 9 (8%) | 7 (7%) | 2 (10%) |
| Splenic aneurysm | 4 (3%) | 4 (4%) | 0 |
| Superior mesenteric artery dissection | 1 (0.9%) | 1 (1%) | 0 |
| Undulating aorta | 4 (3%) | 4 (4%) | 0 |
| Pelvic | 32 (28%) | 30 (32%) | 2 (10%) |
| Iliac FMD | 22 (19%) | 21 (22%) | 1 (5%) |
| Iliac dissection | 4 (3%) | 4 (4%) | 0 |
| Iliac dilatation | 9 (8%) | 9 (9%) | 0 |
| Intracranial ∗ | 14 (35%) | 12 (43%) | 2 (17%) |
| Intracranial aneurysm | 9 (23%) | 7 (25%) | 2 (17%) |
| Intracranial FMD | 3 (8%) | 3(11%) | 0 |
| Intracranial vessel irregularity/tortuosity | 2 (5%) | 1 (4%) | 1 (8%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


