The objective of this study was to investigate the prevalence, electrophysiologic properties, and clinical implications of dissociated pulmonary vein (PV) activity after PV antrum isolation (PVAI) in patients with paroxysmal atrial fibrillation (AF). One hundred seventy-three consecutive patients (61 ±10 years old, 141 men) with drug-refractory paroxysmal AF who underwent AF ablation were analyzed. After identification of arrhythmogenic foci, PVAI was performed in all patients. Of the total 346 isolated ipsilateral PVs, 97 (28.0%) were silent, 35 (10.1%) demonstrated isolated ectopic beats, 209 (60.4%) demonstrated a regular ectopic rhythm, and 5 (1.4%) demonstrated fibrillatory activity. The culprit thoracic vein was identified in 77 patients (44.5%). After isolation of ipsilateral PVs, venous activity was observed in 68 (79.1%) and 178 (68.5%) PVs among the 86 PVs with AF triggers and 260 PVs without AF triggers, respectively (p = 0.06). There was no significant difference in the incidence of acute PV reconnections exposed by adenosine triphosphate between the 97 silent ipsilateral PVs and 209 ipsilateral PVs with dissociated PV activity after the PVAI (20.6% vs 19.1%, p = 0.78). After a mean follow-up of 48.7 ± 7.9 months there was no significant difference in rates of freedom from atrial tachyarrhythmias after a single procedure between patients with and those without dissociated activity (62.1% vs 63.3%, p = 0.74, log-rank test). In conclusion, although dissociated PV activity appearing after PV isolation is an important electrophysiologic finding to prove bidirectional conduction block between the left atrium and the PV during the procedure, the clinical implications might be limited.
Since it was reported that most paroxysmal atrial fibrillations (AFs) are triggered from the pulmonary veins (PVs), radiofrequency catheter ablation of AF has become an important therapy with good procedural success rates. Several ablation techniques have been developed; however, electrical PV isolation is the cornerstone of AF ablation, especially for patients with paroxysmal AF. PV antrum isolation (PVAI) has become a widely accepted strategy and superior to segmental PV isolation with regard to success rate. During the AF ablation procedure dissociated PV electrical activity is frequently observed after achieving PV electrical isolation. Recent published data have demonstrated that most patients demonstrate dissociated activity in ≥1 PV after PV isolation. However, the clinical implication of dissociated PV activity remains unclear. The objective of this study was to investigate the prevalence, electrophysiologic properties, and clinical implications of dissociated PV activity after PVAI in patients with paroxysmal AF.
Methods
This study consisted of 173 consecutive patients with drug-refractory symptomatic paroxysmal AF (61 ±10 years old, 141 men) who underwent AF ablation in our hospital. AF was defined as paroxysmal when AF terminated spontaneously and lasted <7 days according to the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society 2007 consensus statement on catheter and surgical ablation of AF and patients with persistent AF were not included. All patients gave their written informed consent to participate in the study.
All antiarrhythmic drugs were discontinued for >7 days (amiodarone was discontinued >1 month) before the ablation procedure. All patients were effectively anticoagulated for >1 month. Transesophageal echocardiography was performed to exclude any atrial thrombi. Surface electrocardiogram and bipolar intracardiac electrograms were continuously monitored and stored on a computer-based digital recording system (Cardiolab System, Prucka Engineering, Houston, Texas; or LabSystem PRO, Bard Electrophysiology, Lowell, Massachusetts). Bipolar electrograms were filtered from 30 to 500 Hz. A 7Fr 20- or 14-pole 2-site mapping catheter (Irvine Biomedical, Inc., Irvin, California) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. Electrophysiologic study was performed under sedation with propofol or dexmedetomidine.
The ablation strategy has been described previously in detail. In brief, after a transseptal puncture a long sheath (SR0, AF Division, St. Jude Medical, Minneapolis, Minnesota) and a Mullins transseptal sheath (Medtronic, Corp., Minneapolis, Minnesota) were introduced into the left superior PV and left inferior PV, respectively. Left pulmonary venography and contrast esophagography were simultaneously performed to obtain the anatomic relation of the PV ostia to the esophagus. Subsequently, right pulmonary venography was undertaken. Heparin 5,000-IU intravenous bolus was administered after the transseptal puncture, with a continuous infusion of 1,000 IU/hour to maintain an activated clotting time of 200 to 300 seconds.
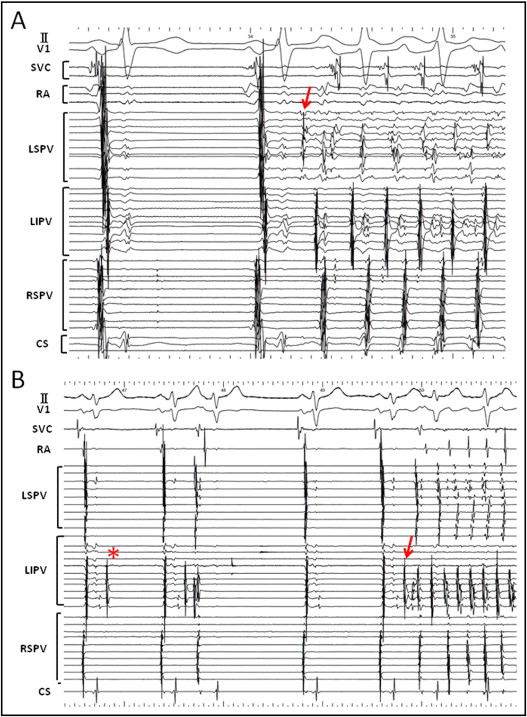
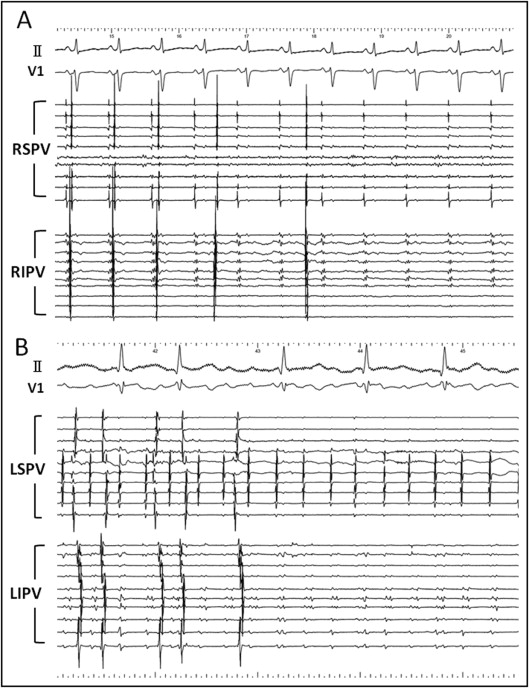
Before starting the ablation 2 or 3 mapping catheters were placed in each PV or superior vena cava (SVC) and isoproterenol was infused. When spontaneous firing was not observed in thoracic veins even with isoproterenol, AF was induced by atrial burst pacing from the coronary sinus and terminated by intracardiac defibrillation. When the trigger of AF was identified in the thoracic veins, we defined it as an arrhythmogenic vein ( Figure 1 ) . Subsequently, 2 circular mapping catheters (Lasso, Biosense Webster, Diamond Bar, California) were placed in the superior and inferior PVs and the left- and right-sided ipsilateral PVs were circumferentially and extensively ablated under fluoroscopic and electrophysiologic guidance. The left atrial posterior wall, at a distance of 1 to 3 cm from the left- or right-sided ostia of the PVs, was anatomically ablated and distal edges of the anterior aspect of the PVs with early PV potentials or continuous PV and left atrial potentials were targeted for ablation. Isolation of left-sided PVs was performed during distal coronary sinus pacing and isolation of right-sided PVs during sinus rhythm. Radiofrequency current deliveries were applied with an 8-mm–tip ablation catheter (Japan Lifeline, Inc., Tokyo, Japan) in the temperature-control mode with a target temperature of 55°C and maximum power of 35 W on the left atrial posterior wall and 40 W at the anterior aspect of the PVs. A deflectable 4-mm–tip ablation catheter connected to a thermocouple thermometer (Delta Ohm, Caselle di Selvazzano, PD, Italy) was used for an esophageal temperature probe. Radiofrequency energy delivery was applied for 40 seconds per point on the left atrial posterior wall; however, if the esophageal temperature reached 42°C, the application was stopped and restarted when it was <38°C. The end point was elimination of all PV potentials ( Figure 2 ) .
After each ipsilateral PV was isolated the electrical activity was assessed using the 2 circular mapping catheters in the ipsilateral PVs for ≥5 minutes. Rhythms in the PV were classified the same as in a previous report : (1) silent, if there was no electrical activity; (2) isolated ectopic beats, if there were intermittent potentials without a regular rhythm; (3) regular ectopic rhythm; or (4) fibrillatory activity. If >1 rhythm was noted in the PV during the observation period, the predominant rhythm was recorded. After completing PVAI, adenosine triphosphate (ATP) 40-mg bolus was injected to unmask any dormant PV conduction and additional radiofrequency was applied as required. After PVAI a cavotricuspid isthmus line was created with an end point of bidirectional conduction block. In cases in which ectopic foci within the SVC initiated AF, the SVC was isolated.
After 3 to 6 months, in the absence of any AF, anticoagulation was discontinued. No antiarrhythmic drugs were prescribed after the procedure. Patients underwent continuous electrocardiographic monitoring as inpatients for 3 days after the procedure. Patients were seen at 2, 6, 10, 14, 24, 36, and 48 weeks after discharge and every 3 months thereafter with Holter monitoring. Patients with palpitations were encouraged to use an event recorder. Recurrent AF was defined according to a patient’s symptoms and/or if AF lasting >30 seconds was seen on electrocardiogram. A blanking period of 1 month was used and a repeat ablation procedure was recommended for patients developing AF/atrial tachycardia after this period.
Continuous variables are expressed as mean ± SD or median and interquartile range depending on normality of the distribution. Continuous and categorical variables were compared using Student’s t test and chi-square test, respectively. A probability value <0.05 indicated statistical significance. Kaplan–Meier analysis was used to determine the percentage of patients free from AF after the initial procedure and any differences in AF-free survival were evaluated using log-rank test.
Results
Baseline clinical and echocardiographic preprocedural characteristics are listed in Table 1 . Five patients had cardiomyopathy (dilated cardiomyopathy in 2 patients and hypertrophic cardiomyopathy in 3), 16 had coronary artery disease, and 15 had valvular heart disease.
| Age (years) | 60.6 ± 10.1 |
| Men | 141 (81%) |
| Atrial fibrillation duration (months) | 59.9 ± 62.3 |
| Ineffective antiarrhythmic drugs | 2.4 ± 1.7 |
| Structural heart disease | 34 (20%) |
| Left atrial diameter (mm) | 38.5 ± 5.5 |
| Left ventricular ejection fraction (%) | 65.6 ± 8.8 |
| Left ventricular diastolic diameter (mm) | 48.6 ± 4.8 |
Successful PVAI with bidirectional conduction block of the cavotricuspid isthmus was achieved in all patients. Among 346 isolated ipsilateral PVs, 229 ipsilateral PVs (66.2%, 122 in the left and 107 in the right) were isolated simultaneously including 9 left common PVs ( Figure 2 ). SVC isolation was added in 5 patients (2.9%). After a mean follow-up of 48.7 ± 7.9 months 111 patients (64.2%) were free of AF recurrences without any antiarrhythmic drugs after a single ablation procedure.
After PVAI 151 patients (87.2%) had dissociated activity in ≥1 isolated ipsilateral PV ( Figure 3 ) . Of the total 346 isolated ipsilateral PVs 97 (28.0%) were silent, 35 (10.1%) demonstrated isolated ectopic beats, 209 (60.4%) demonstrated a regular ectopic rhythm, and 5 (1.4%) demonstrated fibrillatory activity. Among PVs with a regular ectopic rhythm average cycle length was 2,320 ± 1,403 ms (median 1,800, interquartile range 1,400 to 2,975). Cycle length remained steady or increased over the recording time after PVAI in most cases. Of 5 patients who underwent SVC isolation dissociated rhythm and SVC fibrillation were observed in 1 patient (20%) after SVC electrical isolation ( Figure 4 ) .
Dissociated PV activity was compared based on side of the PV. Of the 173 patients included in this study PV activity was seen in 124 (71.7%) and 125 (72.3%) of the right and left ipsilateral PVs, respectively (p = 0.90). Eighty patients (46.2%) had dissociated PV activity bilaterally, whereas 22 patients (12.7%) did not have electrical activity in any vein after isolation.
Dormant left atrial–PV conduction was exposed by ATP injection in 58 (16.8%) ipsilateral PVs transiently and in 10 (2.9%) ipsilateral PVs persistently ( Figure 5 ) . Dormant conduction was exposed significantly more frequently in left ipsilateral PVs than in right ipsilateral PVs (43 veins, 24.9%, vs 25 veins, 14.5%, p = 0.01). There was no significant difference in the rate of acute PV reconnections exposed by ATP between the 97 silent ipsilateral PVs and 209 ipsilateral PVs with dissociated PV activity after PVAI (20.6% vs 19.1%, p = 0.78). All dormant left atrial–PV reconnections were eliminated by additional radiofrequency applications.




