The administration of antiplatelet drugs for months after a drug-eluting stent implantation is critical in decreasing the risk of complications, and premature discontinuation of antiplatelet therapy before the recommended period is the most important predictor for late complications. Therefore, we investigated the prevalence and associated factors of premature discontinuation of antiplatelet therapy in patients in Korea. This retrospective cohort study was conducted using the Korean National Health Insurance Service–National Sample Cohort data. Patients who were treated with dual-antiplatelet therapy (DAPT) were identified with medication prescription data. The Kaplan–Meier failure time plot was used to illustrate the cumulative probability of treatment discontinuation. Cox regression analysis was conducted to compare predictors of early discontinuation of DAPT. The characteristics of the early discontinuation group were not significantly different from the guideline concordance group, except for a higher prevalence of disability and a lower rate of chronic kidney disease. In a Cox regression model, the presence of hypertension was identified as a negative predictor of early discontinuation, and disability was not a statistically significant predictor. The prevalence of early discontinuation was 31.0% and seems to be significantly higher than those reported from prospective studies, which may more accurately reflect the real-world situation. In conclusion, physicians should make more effort to educate patients on the risk associated with premature discontinuation of antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent, and further studies investigating the reasons for nonadherence of DAPT are needed to improve DAPT compliance.
According to guidelines published by the American College of Cardiology (ACC), the American Heart Association (AHA), and the Society of Cardiovascular Angiography and Interventions (SCAI) since 2007, the indefinite use of aspirin and “at least 12-month use” of clopidogrel, that is, a minimum 12-month administration of dual-antiplatelet therapy (DAPT), have been recommended for patients treated by percutaneous coronary intervention (PCI) with a drug-eluting stent (DES). Premature discontinuation of DAPT before the recommended period is the most important predictor for complications after DES implantation, such as stent thrombosis, myocardial infarction, and death. Depending on factors related to study design, prevalence of early discontinuation of antiplatelet therapy has varied in previous studies, where rates of aspirin adherence, mostly >90%, and rates of clopidogrel adherence, varying from 36% to 90%, over a 12-month period were reported. Most previous studies were designed prospectively with the period of data collection before 2007, and risk factors associated with nonadherence were not consistent. A small number of retrospective studies have been conducted and showed a tendency of low rates of DAPT adherence compared to those reported in prospective studies, especially, with patient self-reporting data. To our knowledge, there have been only a few studies about DAPT adherence after PCI with DES in Korea, which were also prospectively conducted with patient self-reporting data. In this study, we sought to investigate prevalence of premature discontinuation of antiplatelet therapy within 12 months after DES implantation in patients in Korea, after publication of the 2007 guideline. We also aimed to determine the role of baseline clinical and socioeconomic factors in predicting the compliance of patients to prolonged DAPT after DES implantation, which might help set strategies to improve adherence to DAPT in a risky group of early discontinuation.
Methods
This research was conducted using the Korean National Health Insurance Service–National Sample Cohort (2002∼2013; NHIS-NCS) data. The NHIS-NCS data contain all medical and demographic information of 2% of randomly selected people (amounting to approximately 1 million people in total) from the total Korean population (about 50 million) as of January 1, 2002. In Korea, the NHIS provides mandatory universal health insurance to nearly all Koreans (97%). Medical information includes utilization of medical facilities, such as clinic visits as outpatients and hospitalization, disease code registered by clinicians, and prescribed medicines, including dates of prescription and amount dispensed. Demographic information includes age, gender, insurance premium (proxy for income level), disability status, and place of residence. The Korean Ministry of Health and Welfare runs the national registration system of people with disabilities, including information about the degree of disability, which is evaluated by functional status of disability. Types of disability, such as learning disability, physical disability, and visual impairment, also could be found in the system. Numbers of patients for each type of disabilities, however, were too small to be analyzed separately; therefore, it was treated as 1 variable, presence of disability, in this study.
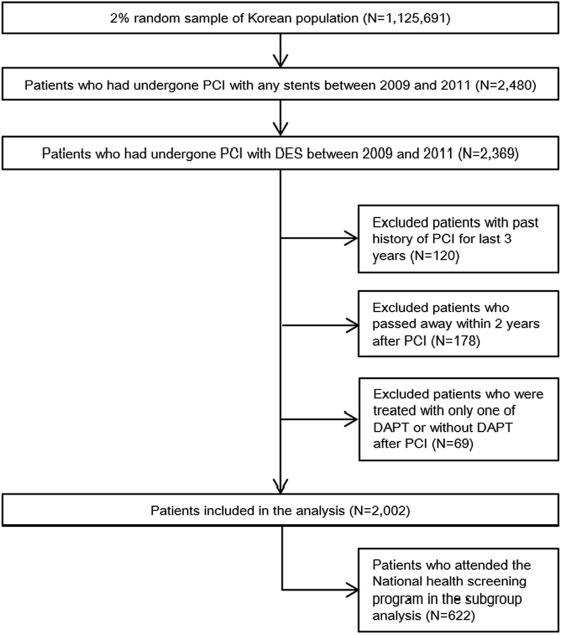
Patients who were treated with DES were identified by the medical material codes from hospital-claimed bills to NHIS to reimburse medical expenses. The study population was restricted to registered patients from January 1, 2009, to December 31, 2011. The reason for setting 2009 as the starting year was that the clinical guideline published in 2007 recommended 12-month DAPT administration after DES insertion as the mandatory period, and it was assumed that 2 years would be enough for this guideline to be adopted universally in the clinical field. A 2-year follow-up period after DES insertion was also set up to investigate prescription patterns and compliance of antiplatelet therapy beyond the recommended duration. In the target population, patients who had a history of stent implantation for the past 3 years were excluded to minimize any confounding effects of DAPT administration because of previous experience of stent insertion. Patients who passed away in the follow-up period were also excluded. Patients who attended the national health screening program at the year of DES implantation were included in the subgroup analysis to further investigate the influence of health behaviors (smoking, drinking, physical activity) on DAPT adherence (screening subgroup).
Patients who were treated with DAPT after PCI with DES were identified with medication prescription data. Patients need to get a prescription from a clinician every time to refill medication in Korea. If a patient had a record of prescriptions for aspirin and clopidogrel in the documentation of hospitalization for PCI or if a patient was prescribed aspirin and clopidogrel after discharge and the term of the first date of prescriptions between aspirin and clopidogrel was <30 days, the patients were considered to be treated with DAPT and was included in the analysis. Time to discontinuation of antiplatelet drugs was defined as the last date of a drug prescription in the 2 years after an individual patient was treated with DES. The last prescription date of aspirin and clopidogrel was identified separately. If both of aspirin and clopidogrel or 1 of the 2 agents was discontinued in 1 year after the intervention (i.e., ≤358 days, allowing 7-day window), it was defined as early discontinuation of DAPT after PCI with DES. We also calculated discontinuation within 6 months to further evaluate compliance to recent evidence that administration of DAPT for 6 months only was not inferior to the current 12-month protocol.
Characteristics of patients who discontinued DAPT within 1 year (the early discontinuation group) were compared with those who continued to use it for over a year (the guideline concordance group). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as percentages. Comparisons between groups were done using the t test for continuous variables and chi-square test or Fischer’s exact test for categorical values in univariate analysis. The Kaplan–Meier failure time plot was used to illustrate the cumulative probability of treatment discontinuation after DES insertion. Cox regression analysis also was conducted separately to compare predictors of early discontinuation of DAPT. Demographic factors (such as age, residential area, and income level) and clinical factors (such as Charlson Comorbidity Index and presence of co-morbidities) were included in the model as potential predictors. Health behavior factors, such as smoking, drinking, and physical activity, were additionally included in subgroup analysis. All analysis was conducted with STATA version 14 for Windows (College Station, Texas). A p value <0.05 was considered statistically significant.
Results
A total of 2,369 patients who underwent DES implantation from January 1, 2009, to December 31, 2011, were identified in the NHIS-NCS data. After excluding patients who had the past history of stent implantation for the past 3 years, who passed away by 2 years after DES implantation, and who did not start DAPT after DES implantation, a total of 2,002 patients remained and were included in the analysis ( Figure 1 ). The number of patients who experienced DES implantation increased year by year during the period of investigation ( Table 1 ). A total of 622 subjects who had the health screening data were included in subgroup analysis ( Figure 1 ). The prevalence of premature discontinuation of DAPT within 1 year during 2009 to 2011 was 31.0%, and the discontinuation of each of aspirin and clopidogrel in 1 year was 15.4% and 24.5%, respectively ( Table 1 ). Corresponding figures for 6 months were 8.7, 8.0, and 8.2%, respectively. The cumulative probability of treatment discontinuation is presented in Figure 2 .