Although coronary computed tomographic angiography has the ability to depict potentially malignant features of anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS), there are limited data on the significance of ACAOS in the computed tomography population. The aims of this study were to assess the prevalence of ACAOS and to correlate its anatomic features with patients’ symptoms among 8,522 consecutive subjects who underwent coronary computed tomographic angiography from February 2008 to May 2012. The ACAOS proximal course was classified into anterior, interarterial, septal, and retroaortic subtypes. Malignant ACAOS was recorded if a slitlike ostium, an acute angle of takeoff, an intramural course, and significant compression between the aorta and pulmonary trunk were present simultaneously. The prevalence of ACAOS was 0.84% (72 of 8,522), including right-sided origins of the left main coronary artery (n = 11), left anterior descending coronary artery (n = 9), and left circumflex coronary artery (n = 33) and left-sided origin of the right coronary artery (n = 20). Of the 24 ACAOS (0.28%) with an interarterial course, 12 (0.14%) showed significant vessel compression, of which 6 (0.07%) were classified as malignant. The presence of significant interarterial compression and malignant ACAOS type were observed in left-sided right coronary arteries only, and interarterial compression correlated with patients’ symptoms at a median of 15-month follow-up. In conclusion, the computed tomographic prevalence of ACAOS seems to be comparable with that of previous angiographic studies. The malignant features of ACAOS in the adult computed tomography population might be exclusively associated with left-sided right coronary arteries.
Anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS) is a rare congenital anomaly that accounts for almost 30% of sudden cardiac deaths in young adults and competitive athletes. Data from necropsy and intravascular ultrasound studies have shown the possible relation between the anatomic characteristics of ACAOS and worse clinical outcomes. Although coronary computed tomographic angiography (CCTA) has the potential to identify malignant anatomic features of ACAOS, there are limited data on the prevalence and clinical significance of ACAOS in an adult computed tomography population. The aims of the present study were (1) to assess the prevalence and anatomic characteristics of ACAOS, and (2) to retrospectively correlate anatomic features of ACAOS with patients’ symptoms and clinical prognoses in a population referred for CCTA at a single high-volume center.
Methods
We reviewed retrospectively a database of 8,928 consecutive patients who were referred for CCTA from February 2008 to May 2012 to the Institute of Cardiology in Warsaw, Poland. The computed tomographic reports were analyzed for the presence of ACAOS, and those with (1) right-sided origin of the left main coronary artery (LMCA), (2) right-sided origin of the left anterior descending coronary artery (LAD), (3) right-sided origin of the left circumflex artery (LC), and (4) left-sided origin of the right coronary artery (RCA) were selected for further assessment. Of a total of 8,928 patients, 406 (4%) failed to undergo contrast-enhanced CCTA because of high calcium scores (n = 289), intended noncontrast calcium score assessment (n = 52), significant arrhythmias (n = 48), lack of peripheral venous access or contrast extravasation (n = 9), patient refusal (n = 5), and contrast intolerance (n = 3). The final analysis was thus performed on 8,522 patients. No computed tomographic study with ACAOS was excluded because of poor image quality. Anomalous coronary origin from the noncoronary sinus or the pulmonary trunk, including complex congenital heart defects, was excluded. We reviewed clinical records and obtained follow-up data by telephone interviews and through an electronic database. The institutional ethics committee approved the study, and all patients gave informed consent.
Studies conducted from February 2008 to July 2011 (5,534 patients [65%]) were performed using a first-generation dual-source computed tomographic scanner (Somatom Definition; Siemens Healthcare, Forchheim, Germany), with beam collimation of 64 × 0.6 mm, a gantry rotation time of 330 ms, tube voltage of 80 to 140 kV, pitch of 0.2 to 0.3, and temporal resolution of 83 ms. Unless contraindicated, an intravenous bolus of metoprolol (sequential 2.5 mg doses) was administered to target a heart rate <65 beats/min, and sublingual nitroglycerin (0.8 mg) was given directly before computed tomography. The computed tomographic protocol was extended to the measurement of a coronary artery calcium score if clinically indicated. A retrospective or prospective electrocardiographically gated acquisition protocol was used at the operator’s discretion. To minimize radiation exposure, electrocardiographically gated tube current modulation was applied in all patients. Scan data were reconstructed routinely in mid- to end-systole and diastole (35% to 45% and 65% to 75% of the RR interval). Studies conducted from August 2011 to May 2012 were performed using a second-generation dual-source computed tomographic scanner (Somatom Definition Flash; Siemens Healthcare) using the same protocol (n = 2,093 [25%]) or a high-pitch Flash protocol (n = 895 [10%]), with beam collimation of 64 × 0.6 mm, a gantry rotation time of 280 ms, tube voltage of 80 to 120 kV, pitch of 3.2 to 3.4, and temporal resolution of 75 ms. Image acquisition was prospectively triggered by the patient’s electrocardiogram using a high-pitch spiral protocol, and the data acquisition started at 55% to 60% of the R peak–to–R peak interval. Slice thickness was 0.6 mm, with an increment of 0.4 mm, irrespective of the computed tomographic protocol, and the contrast injection technique was similar for all studies. For acquisition of the volume data set, a bolus of 60 to 120 ml iodinated contrast material (Iomeron 400; Bracco Altana Pharma, Konstanz, Germany) was administered through an antecubital vein at a rate of 6 ml/s. The average effective radiation doses were determined on the basis of the recommended conversion factors as previously described.
All selected computed tomographic scans were analyzed off-line on a dedicated workstation (Leonardo; Siemens Healthcare) by a consensus of 2 experienced observers blinded to all patient data. The origin and course of ACAOS were evaluated using multiplanar reconstructions and 3-dimensional volume-rendered images. The proximal ACAOS course was classified into the following pathways: anterior (prepulmonary), interarterial (between the aorta and the pulmonary artery), septal (posterior to the right ventricular outflow tract within the myocardium of interventricular septum), and retroaortic. Malignant ACAOS was defined if all of the following anatomic features were recorded, as previously described : a slitlike ostium (≥50% luminal narrowing of coronary orifice), an acute angle of takeoff (defined as an angle ≤30° between the plane longitudinal to the proximal ACAOS and the tangential plane of the aortic root at the level of the ACAOS in axial multiplanar reconstruction), a proximal intramural course within the aortic wall, and significant lateral compression (≥50% luminal narrowing) between the aorta and the pulmonary trunk in the diastolic and/or systolic phase of the heart cycle. Additionally, we assessed the presence of significant systolic compression of ACAOS running intraseptally. Significant coronary artery disease was defined as ≥50% luminal stenosis by visual assessment in ≥1 major coronary artery on CCTA.
Continuous data are reported as mean ± SD and categorical data as frequencies and percentages. Nonparametric Mann-Whitney tests were used to assess differences between continuous variables. Differences in categorical data were analyzed using Fisher’s exact test. Significance was defined as a p value <0.05, and analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, Illinois).
Results
Of the 8,522 subjects, 7,447 (87%) and 1,075 (13%) underwent computed tomography because of suspected and known coronary artery disease, respectively. The median age was 60 ± 11 years (range 12 to 93), and 4,222 patients (49%) were male.
The prevalence of ACAOS was 0.84% (72 of 8,522). Among the 72 patients, there was a total of 73 ACAOS (1 patient had 2 coronary anomalies: LAD and LC originating separately from the right aortic sinus). Clinical characteristics are listed in Table 1 . The median age was 55 ± 12 years (range 23 to 85), and 37 patients were men (51%). Thirty-eight patients (53%) underwent CCTA because of atypical chest pain, whereas 32 patients (44%) had typical angina. Two patients (3%) were admitted with diagnoses of acute coronary syndromes, both of whom had left-sided RCAs with significant interarterial compression and slitlike ostia (the first patient had normal coronary arteries and an intramural RCA course, whereas the second patient had 2-vessel disease with distal occlusion of the LAD and a significant stenosis in the proximal LC). There were no differences in gender distribution (51% vs 49% male, p = 0.813) or the prevalence of significant coronary artery disease as detected by CCTA (29% vs 34%, p = 0.385) between patients with and without ACAOS. In contrast, patients with ACAOS were significantly younger compared with those without ACAOS (55 ± 12 vs 60 ± 11 years, p = 0.002).
| Variable | Total (n = 72) | LMCA from RSV (n = 11) | LAD from RSV (n = 9) | LC from RSV (n = 32) | RCA from LSV (n = 20) |
|---|---|---|---|---|---|
| Age (yrs) | 55 ± 12 | 59 ± 11 | 64 ± 13 | 53 ± 12 | 54 ± 12 |
| Men | 37 (51%) | 6 (54%) | 5 (56%) | 14 (44%) | 12 (60%) |
| Diabetes mellitus | 9 (12%) | 5 (45%) | 1 (11%) | 1 (3%) | 2 (10%) |
| Hypertension ∗ | 58 (81%) | 10 (91%) | 7 (78%) | 26 (81%) | 15 (75%) |
| Hyperlipidemia † | 49 (68%) | 6 (54%) | 7 (78%) | 22 (69%) | 14 (70%) |
| Current smokers | 14 (19%) | 3 (27%) | 2 (22%) | 4 (12%) | 5 (25%) |
| Atypical chest pain | 38 (53%) | 4 (36%) | 5 (56%) | 18 (56%) | 11 (55%) |
| Stable angina pectoris | 32 (44%) | 7 (64%) | 4 (44%) | 14 (44%) | 7 (35%) |
| Canadian Cardiovascular Society class I/II | 16 (22%) | 2 (18%) | 2 (22%) | 10 (31%) | 2 (10%) |
| Canadian Cardiovascular Society class III/IV | 16 (22%) | 5 (45%) | 2 (22%) | 4 (12%) | 5 (25%) |
| Acute coronary syndromes | 2 (3%) | 0 | 0 | 0 | 2 (10%) |
| Syncope ‡ | 26 (36%) | 5 (45%) | 4 (44%) | 10 (31%) | 7 (35%) |
| Palpitations | 26 (36%) | 6 (54%) | 3 (33%) | 12 (37%) | 5 (25%) |
| Atrial fibrillation | 19 (26%) | 6 (54%) | 3 (33%) | 7 (22%) | 3 (15%) |
| Ventricular arrhythmia | 11 (15%) | 2 (18%) | 0 | 6 (19%) | 3 (15%) |
| Family history of sudden death § | 20 (28%) | 2 (18%) | 3 (33%) | 7 (22%) | 8 (40%) |
| Previous myocardial infarction | 10 (14%) | 2 (18%) | 1 (11%) | 4 (12%) | 3 (15%) |
| Previous myocardial infarction with normal coronary arteries | 3 (4%) | 0 | 0 | 2 (6%) | 1 (5%) |
| Previous heart failure | 11 (15%) | 2 (18%) | 2 (22%) | 4 (12%) | 3 (15%) |
| Previous coronary stenting | 2 (3%) | 1 (9%) | 0 | 1 (3%) | 0 |
| Previous coronary artery bypass grafting | 2 (3%) | 0 | 0 | 0 | 2 (10%) |
| Aortic valvular disease | 5 (7%) | 1 (9%) | 1 (11%) | 1 (3%) | 2 (10%) |
| Coarctation of aorta | 2 (3%) | 0 | 0 | 1 (3%) | 1 (5%) |
| Coronary angiography before CCTA | 18 (25%) | 7 (64%) | 2 (22%) | 3 (9%) | 5 (25%) |
| Coronary angiography nondiagnostic | 10 (14%) | 6 (54%) | 1 (11%) | 1 (3%) | 2 (10%) |
| Coronary computed tomographic angiographic characteristics | |||||
| Significant coronary artery disease | 21 (29%) | 3 (27%) | 5 (56%) | 8 (25%) | 5 (25%) |
| Mean coronary calcium score | 166 ± 333 | 159 ± 206 | 259 ± 296 | 104 ± 217 | 227 ± 513 |
| Coronary calcium score = 0 | 24 (33%) | 2 (18%) | 2 (22%) | 11 (34%) | 9 (45%) |
| Signal-to-noise ratio | 21.5 ± 6.2 | 22.0 ± 5.1 | 23.2 ± 3.1 | 21.3 ± 7.4 | 20.7 ± 6 |
| Contrast-to-noise ratio | 22.8 ± 6.6 | 23.1 ± 6.1 | 24.4 ± 3.6 | 22.5 ± 7.6 | 22.2 ± 6.7 |
| Dose-to-length product (mGy . cm) | 697 ± 340 | 687 ± 370 | 645 ± 300 | 705 ± 390 | 700 ± 380 |
| Effective dose (mSv) | 9.8 ± 4.8 | 9.6 ± 5.2 | 9 ± 4.2 | 9.9 ± 5.5 | 9.8 ± 5.3 |
∗ Use of antihypertensive drugs and/or blood pressure ≥140/90 mm Hg.
† Use of lipid-lowering drugs and/or total serum cholesterol ≥200 mg/dl.
‡ Transient loss of consciousness and postural tone characterized by rapid onset, short duration, and spontaneous recovery.
§ Family history of sudden death in a first-degree relative.
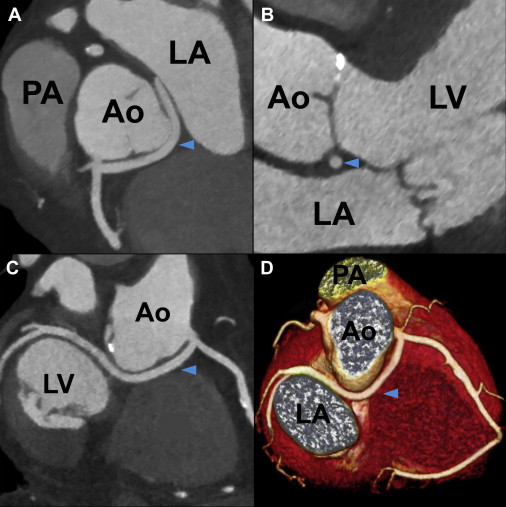
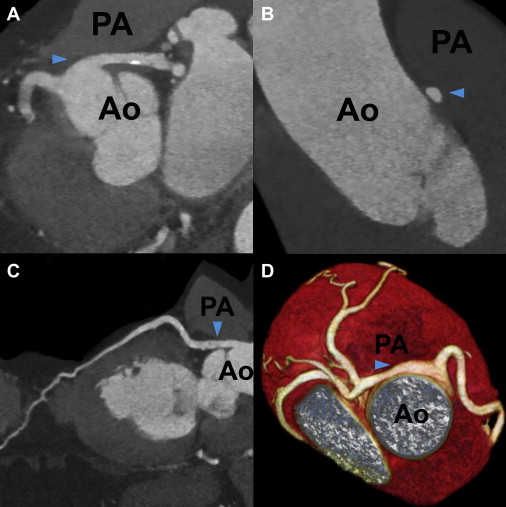
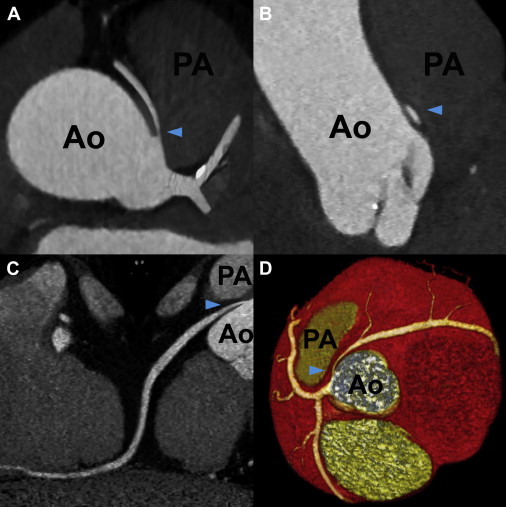
Right-sided LC was the most common ACAOS in our population, followed by left-sided RCA, with computed tomographic prevalence of 0.39% (33 of 8,522) and 0.23% (20 of 8,522), respectively. The proximal course and potentially malignant anatomic characteristics of ACAOS are listed in Table 2 . Anomalous LMCAs and LADs showed most commonly intraseptal courses, followed by anterior and interarterial courses. There were neither LMCA nor LAD cases running posterior to the aorta. In contrast, anomalous LCs ( Figure 1 ) showed retroaortic courses in 32 of 33 patients (97%), and the RCA always took an interarterial course. None of the anomalous LMCAs or LADs with anterior courses showed malignant anatomic features. Although LMCAs and LADs with septal courses lacked slitlike ostia or intramural aortic courses, we identified 2 LADs (22%) with significant compression within the myocardium during systole. Of the 32 LCs with retroaortic courses, only 2 (6%) showed intramural courses and acute angles of takeoff. Of the 24 ACAOS (0.28%) with interarterial courses, most of 20 cases (0.23%) were attributed to left-sided RCAs, of which 12 (0.14%) showed significant interarterial compression. In contrast to anomalous RCAs, none of the LMCAs ( Figure 2 ), LADs, or LCs with interarterial courses revealed significant vessel compression, slitlike ostia, or intramural aortic courses, and RCAs ( Figure 3 ) showed a significantly higher prevalence of malignant anatomic features (62% vs 12%, p <0.001) compared with the remaining ACAOS with interarterial courses. We identified 6 anomalous RCAs demonstrating all 4 malignant features of the proximal vessel course but no associated coronary artery disease ( Table 3 ).
| Variable | LMCA from RSV (n = 11) | LAD from RSV (n = 9) | LC from RSV (n = 33) | RCA from LSV (n = 20) |
|---|---|---|---|---|
| Anterior course | 4 (36%) | 3 (33%) | 0 | 0 |
| Slitlike ostium | 0 | 0 | — | — |
| Acute angle of takeoff | 0 | 0 | — | — |
| Intramural course | 0 | 0 | — | — |
| Interarterial course | 2 (18%) | 1 (11%) | 1 (3%) | 20 (100%) |
| Slitlike ostium | 0 | 0 | 0 | 13 (65%) |
| Acute angle of takeoff | 1 (9%) | 0 | 1 (3%) | 15 (75%) |
| Intramural course | 0 | 0 | 0 | 10 (50%) |
| Significant lateral compression | 0 | 0 | 0 | 12 (60%) |
| Septal course | 5 (46%) | 5 (56%) | 0 | 0 |
| Slitlike ostium | 0 | 0 | — | — |
| Acute angle of takeoff | 2 (18%) | 1 (11%) | — | — |
| Intramural course | 0 | 0 | — | — |
| Significant systolic compression | 0 | 2 (22%) | — | — |
| Retroaortic course | 0 | 0 | 32 (97%) | 0 |
| Slitlike ostium | — | — | 0 | — |
| Acute angle of takeoff | — | — | 2 (6%) | — |
| Intramural course | — | — | 2 (6%) | — |
| Patient | ACAOS | Age (yrs) | Gender | Symptoms at Referral | Slitlike Ostium | Acute Angle of Takeoff | Intramural Course | Significant Compression | 4 Malignant Features Present |
|---|---|---|---|---|---|---|---|---|---|
| 1 | RCA | 27 | M | Atypical chest pain | + | + | 0 | + | 0 |
| 2 | RCA | 35 | M | Atypical chest pain | 0 | + | 0 | 0 | 0 |
| 3 | RCA | 41 | M | Atypical chest pain | + | + | + | + | + |
| 4 | RCA | 42 | F | Atypical chest pain | 0 | + | + | 0 | 0 |
| 5 | RCA | 42 | M | Acute coronary syndrome | + | + | + | + | + |
| 6 | RCA | 48 | M | Atypical chest pain | 0 | + | + | 0 | 0 |
| 7 | RCA | 51 | F | Angina | + | + | + | + | + |
| 8 | RCA | 52 | F | Angina | + | 0 | 0 | + | 0 |
| 9 | RCA | 56 | M | Atypical chest pain | 0 | + | + | 0 | 0 |
| 10 | RCA | 57 | F | Atypical chest pain | + | 0 | 0 | + | 0 |
| 11 | RCA | 58 | M | Atypical chest pain | + | + | + | + | + |
| 12 | RCA | 59 | M | Angina | + | + | 0 | + | 0 |
| 13 | RCA | 59 | F | Angina | + | + | + | + | + |
| 14 | RCA | 61 | M | Acute coronary syndrome | + | + | 0 | + | 0 |
| 15 | RCA | 62 | F | Atypical chest pain | + | 0 | 0 | 0 | 0 |
| 16 | RCA | 64 | M | Angina | + | + | + | + | + |
| 17 | RCA | 65 | M | Atypical chest pain | + | 0 | 0 | + | 0 |
| 18 | RCA | 67 | F | Atypical chest pain | 0 | 0 | + | 0 | 0 |
| 19 | RCA | 69 | F | Angina | 0 | + | 0 | 0 | 0 |
| 20 | RCA | 75 | M | Angina | 0 | + | 0 | 0 | 0 |
| 21 | LMCA | 50 | M | Atypical chest pain | 0 | 0 | 0 | 0 | 0 |
| 22 | LMCA | 74 | F | Atypical chest pain | 0 | + | 0 | 0 | 0 |
| 23 | LAD | 85 | F | Atypical chest pain | 0 | 0 | 0 | 0 | 0 |
| 24 | LC | 56 | M | Angina | 0 | + | 0 | 0 | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


