Young women who require aortic valve replacement need information on the potential cardiac and obstetric complications of pregnancy for the different valve substitutes available. We, therefore, assessed the pregnancy outcomes in women who had received an autograft, homograft, or mechanical valve in the aortic position. Women who were pregnant after surviving aortic valve replacement at our institution from 1987 to 2011 were included. Information on cardiac status and pregnancy outcome was obtained through the hospital medical records and by an extensive patient questionnaire. A total of 40 women experienced 67 pregnancies, of which 55 (82%) were completed pregnancies, 6 (9%) were miscarriages, and 6 (9%) were terminated. Of the 40 women, 18 (45%) had a pulmonary autograft, 13 (32%) a homograft, and 9 (23%) a mechanical valve. The mean age at the first pregnancy was 30.0 ± 5.7 years. No maternal mortality but 1 fetal death (1.8%) and 1 neonatal death (1.8%) occurred. Maternal cardiac complications developed in 13% and obstetric complications in 38% of the completed pregnancies. Heart failure (9%), arrhythmias (7%), hypertension-related disorders (7%), preterm delivery (24%), and small-for-gestational-age infants (15%) were most often encountered. Mechanical valve recipients had the greatest incidence of both cardiac and obstetric complications. In conclusion, pregnancy-associated complications after aortic valve replacement were common, and human tissue valves should be considered in the discussion for the optimal aortic valve substitute in a young woman. However, careful obstetric monitoring is mandatory.
When a young woman requires aortic valve replacement (AVR), it is important to incorporate reliable information on the potential pregnancy complications and pregnancy outcomes when considering the available surgical options. In mechanical valve recipients, complications due to anticoagulation therapy represent a threat for both the mother and her unborn child. Accelerated valve dysfunction due to degeneration could be a point of concern for biological valve substitutes, although more recent studies have reported that pregnancy does not increase structural deterioration or reduce survival. Limited evidence is available on the rate of cardiac and obstetric complications in young women who become pregnant after AVR. Most available information has concerned mechanical—mainly mitral—valve recipients and showed increased risks of anticoagulation-related complications and increased maternal and fetal mortality and morbidity. Reports on pregnancy-related outcomes are also scarce for human tissue valve recipients. Therefore, the aim of the present study was to determine the occurrence of cardiac and obstetric complications in women who experienced a pregnancy after implantation of an autograft, homograft, or mechanical valve in the aortic position at our institution.
Methods
Women who were pregnant after surviving AVR with a pulmonary autograft, homograft, or mechanical valve prosthesis at the Erasmus University Medical Center, were aged ≤50 years at surgery, had undergone AVR from April 1987 to January 2011, and were ≥16 years old at the last clinical follow-up were invited to participate. The institutional review board approved the study protocol (MEC 2010-272), and all patients provided informed consent. All patients who received a human tissue valve substitute at our institution were followed up prospectively (MEC 2000-813). Eligible patients were identified through our prospective cohort study of human tissue valve recipients and through our departmental patient information system.
Information on pregnancy and cardiac status of the patients until January 1, 2011 was obtained from the hospital medical records and a structured patient questionnaire that was completed from December 1, 2010 to September 1, 2011. We collected data on underlying valve etiology at last surgery, hemodynamic diagnosis, previous surgical or interventional procedures, age at surgery, type (and size) of aortic valve substitute, concomitant procedures, interval from surgery to first pregnancy, age at conception, and preconceptional systolic left ventricular function, maximum aortic jet velocity, and peak pulmonary artery pressure.
Pregnancy was defined as positive human chorionic gonadotropin test or obstetric ultrasound findings. Miscarriage was defined as spontaneous loss of pregnancy at <20 weeks of gestation. Information about each completed pregnancy (duration >20 weeks of gestation) included New York Heart Association (NYHA) functional class, medication, physical examination, pregnancy duration, and mode of delivery. For each infant, the gender, birth weight, and Apgar score were registered.
The registered cardiac complications were arrhythmia (symptomatic, sustained, documented arrhythmia), heart failure (requiring treatment), persistent NYHA functional class deterioration (≥1 year postpartum), syncope, thromboembolic complications, aortic dissection, and/or endocarditis. Obstetric complications included pregnancy-induced hypertension (de novo onset of hypertension after ≥20 weeks of gestation), preeclampsia (hypertension and proteinuria), eclampsia (preeclampsia with grand mal seizures), HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, preterm, premature rupture of membranes (membrane rupture <37 weeks’ gestation), premature labor (spontaneous onset of labor <37 weeks’ gestation), postpartum hemorrhage (>1,000 ml), placental abruption, premature delivery (<37 weeks’ gestation), small-for-gestational-age (birth weight <10th percentile), fetal death (≥20 weeks’ gestation), and neonatal death (<30 days postpartum). The incidence of complications and mode of delivery in the present study was compared to data derived from the 2008 Dutch Perinatal Registry. In that registry, the maternal and fetal data of all deliveries occurring in the Netherlands are recorded (about 180,000; 96% complete). It included both home and hospital deliveries and contained information on the presence of cardiovascular disease in the mother (no additional specification) and neonatal congenital defects (cardiac 0.41%; noncardiac 2.38%).
The anticoagulation therapy administered in our institution to mechanical valve recipients was according to our local protocol and was initiated in close collaboration with the hematologist. As soon as a pregnancy was confirmed, acenocoumarol was changed to a weight-adjusted therapeutic dose of low-molecular-weight heparin (LMWH) until the end of the first trimester and when necessary was monitored by measuring the anti-factor Xa levels. Acenocoumarol was then restarted until 36 weeks of gestation. Then, a therapeutic dose of LMWH was given until spontaneous onset of labor or the day before the induction of labor or elective cesarean section. After delivery, LMWH was initiated again, along with acenocoumarol, until 2 consecutive, appropriate international normalized ratio levels were reached.
The normality of the distribution of continuous data was tested with the Kolmogorov-Smirnov test with Lilliefors’ correction. Continuous data are displayed as the mean ± SD or in the case of a skewed distribution, as the median and interquartile range and were compared using the one-way analysis of variance test or the Kruskal-Wallis test. Discrete data are presented as absolute numbers and percentages and were compared using Pearson’s chi-square test or Fisher’s exact test.
Univariate logistic regression analysis was performed to identify possible factors associated with the incidence of pregnancy-related complications. Missing values were imputed by the mean. Age at surgery, maternal age at first pregnancy, valve type, interval from surgery to first pregnancy, duration of pregnancy, cesarean section, preconceptional left ventricular function, maximum aortic jet velocity, and pulmonary artery pressure were considered as covariables in the univariate model for cardiac and obstetric events. For comparison of the event incidence with the general Dutch population, the chi-square test was used. All statistical tests were 2-sided, and p ≤0.05 was considered significant. For data analysis, SPSS, version 17.0, for Windows (SPSS, Chicago, Illinois) was used.
Results
A total of 40 patients experienced ≥1 pregnancy after AVR in our institution ( Table 1 ), with 67 singleton pregnancies. Of these 67 pregnancies, 55 continued >20 weeks (47% male infants) in 35 women. All 6 spontaneous miscarriages were <14 weeks of gestation. Six pregnancies were terminated ( Table 1 ). The only termination of pregnancy for maternal cardiac reason was performed in a mechanical valve recipient with pulmonary hypertension, tricuspid insufficiency, and moderate stenosis of the mechanical prosthesis in the aortic position of 3.3 m/s. One termination was performed in a fetus with spina bifida. No acenocoumarol-associated embryopathies developed. The mode of delivery for the 55 completed pregnancies, differentiated by type of valve substitute, is listed in Table 2 . Figure 1 illustrates the modes of delivery compared to the Dutch general population. No maternal mortality occurred.
| Variable | All (n = 40) | Autograft (n = 18) | Homograft (n = 13) | MP (n = 9) | p Value |
|---|---|---|---|---|---|
| Intervention or surgery before aortic valve replacement | |||||
| 0 | 23 (58%) | 10 (56%) | 9 (69%) | 4 (44%) | 0.46 |
| 1 | 8 (20%) | 2 (11%) | 4 (31%) | 2 (22%) | 0.46 |
| >1 | 9 (23%) | 6 (33%) | 0 | 3 (33%) | 0.07 |
| Diagnosis | |||||
| Aortic stenosis | 15 (38%) | 10 (56%) | 4 (31%) | 0 | 0.02 |
| Aortic regurgitation | 13 (33%) | 3 (17%) | 6 (46%) | 5 (55%) | 0.10 |
| Mixed | 12 (30%) | 5 (28%) | 3 (23%) | 4 (44%) | 0.61 |
| Etiology | |||||
| Congenital | 26 (65%) | 16 (89%) | 8 (62%) | 2 (22%) | <0.01 |
| Rheumatic | 12 (30%) | 2 (11%) | 4 (31%) | 6 (67%) | 0.01 |
| Aneurysm or dissection | 2 (5%) | 0 | 1 (8%) | 1 (11%) | 0.49 |
| Age at last surgery (yrs) | 25.4 ± 7.7 | 21.5 ± 6.6 | 26.9 ± 5.0 | 31.2 ± 9.0 | <0.01 |
| Concomitant procedures | |||||
| None | 28 (70%) | 16 (89%) | 8 (62%) | 4 (44%) | 0.04 |
| Coronary bypass | 3 (8%) | 1 (6%) | 0 | 2 (22%) | 0.23 |
| Mitral valve surgery | 6 (15%) | 0 | 3 (23%) | 3 (33%) | 0.04 |
| Prosthesis size (mm) | — | — | 22 (21–22) | 21 (21–23) | |
| Interval from surgery to first pregnancy (yrs) ∗ | 3.1 (1.6–6.1) | 5.5 (1.8–9.4) | 2.3 (1.4–4.6) | 2.1 (1.5–4.6) | 0.14 |
| Total pregnancies | 67 | 33 | 22 | 12 | 0.39 |
| 1 | 40 (60%) | 18 (55%) | 13 (59%) | 9 (75%) | 0.46 |
| 2 | 20 (30%) | 11 (33%) | 6 (27%) | 3 (25%) | 0.83 |
| 3 | 7 (10%) | 4 (12%) | 3 (14%) | 0 | 0.46 |
| Pregnancy age (yrs) ∗ | |||||
| First (n = 40) | 30.0 ± 5.7 | 27.0 ± 4.1 | 30.2 ± 4.6 | 35.7 ± 5.9 | <0.01 |
| Second (n = 20) | 30.9 ± 4.7 | 30.0 ± 3.9 | 31.9 ± 5.0 | 32.1 ± 8.0 | 0.75 |
| Third (n = 7) | 32.1 ± 5.5 | 32.7 ± 7.0 | 31.3 ± 4.0 | — | 0.86 |
| Preconceptional left ventricular function (n = 66) | |||||
| Good | 64% | 61% | 77% | 50% | 0.18 |
| Moderate | 36% | 39% | 23% | 50% | 0.18 |
| Preconceptional pulmonary artery pressure (mm Hg) (n = 62) | 6 (3–15) | 13 (9–18) | 3 (2–3) | 4 (1–11) | <0.01 |
| Preconceptional maximum aortic jet velocity (m/s) ∗ | |||||
| First pregnancy (n = 38) | 1.78 ± 0.69 | 1.36 ± 0.42 | 1.85 ± 0.60 | 2.60 ± 0.55 | <0.01 |
| Second pregnancy (n = 19) | 1.70 ± 0.54 | 1.41 ± 0.46 | 2.01 ± 0.36 | 2.23 ± 0.38 | 0.01 |
| Third pregnancy (n = 7) | 1.83 ± 0.80 | 1.41 ± 0.48 | 2.39 ± 0.87 | — | 0.23 |
| Completed pregnancies | 55 (82%) | 28 (85%) | 20 (91%) | 7 (58%) | 0.05 |
| Miscarriage | 6 (9%) | 3 (9%) | 0 | 3 (25%) | 0.05 |
| Pregnancy terminated | 6 (9%) | 2 (6%) | 2 (9%) | 2 (17%) | 0.64 |
| Social reasons | 4 (6%) | 2 (6%) | 2 (9%) | 0 | 0.70 |
| Maternal cardiac indication | 1 (1%) | 0 | 0 | 1 (17%) | 0.18 |
| Fetal spina bifida | 1 (1%) | 0 | 0 | 1 (8%) | 0.18 |
∗ All 67 pregnancies, including miscarriages and terminations.
| Variable | All (n = 55) | Autograft (n = 28) | Homograft (n = 20) | MP (n = 7) | p Value |
|---|---|---|---|---|---|
| Vaginal delivery ∗ | 42 (76%) | 19 (68%) | 17 (85%) | 6 (86%) | 0.32 |
| Spontaneous | 11 (20%) | 3 (11%) | 5 (25%) | 3 (43%) | 0.25 |
| Assisted delivery | 13 (24%) | 7 (25%) | 5 (25%) | 1 (14%) | 0.67 |
| Epidural anesthesia | 11 (20%) | 4 (14%) | 5 (25%) | 2 (29%) | 0.80 |
| Induction of labor | 20 (36%) | 11 (39%) | 7 (35%) | 2 (29%) | 0.53 |
| Elective cesarean section | 8 (15%) | 5 (18%) | 2 (10%) | 1 (14%) | 0.89 |
| Maternal cardiovascular risk | 5 (9%) | 3 (11%) | 2 (10%) | 0 | 0.72 |
| Prosthetic valve thrombosis | 1 (2%) | 0 | 0 | 1 (14%) | 0.13 |
| Fetal presentation | 1 (2%) | 1 (4%) | 0 | 0 | 1.00 |
| Fetopelvic disproportion | 1 (2%) | 1 (4%) | 0 | 0 | 1.00 |
| Emergency cesarean section | 5 (9%) | 4 (14%) | 1 (5%) | 0 | 0.42 |
| Fetal distress † | 2 (4%) | 1 (4%) | 1 (5%) | 0 | 1.00 |
| Placental abruption | 1 (2%) | 1 (4%) | 0 | 0 | 1.00 |
| Fetopelvic disproportion | 2 (4%) | 2 (7%) | 0 | 0 | 0.62 |
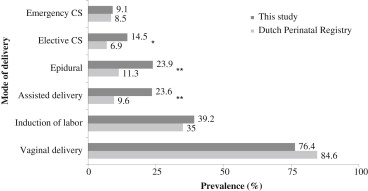
Heart failure was the most common cardiac complication, with persistent NYHA deterioration in 3 patients ( Table 3 ). One mechanical valve recipient with permanent atrial fibrillation developed prosthetic valve thrombosis and subsequent heart failure at 33 weeks’ gestation. Anticoagulation was converted to intravenous heparin, and the woman underwent cesarean section at 36 weeks. A female infant of 2,150 g was born. Five weeks later, she underwent repeat AVR with another mechanical valve.
| Variable | All (n = 55) | Autograft (n = 28) | Homograft (n = 20) | MP (n = 7) | p Value |
|---|---|---|---|---|---|
| Pregnancy duration (weeks) | 38 (36–40) | 38 (35–40) | 39 (38–40) | 36 (31–39) | 0.20 |
| Birth weight (kg) (n = 54) | 3.0 (2.5–3.3) | 3.0 (2.4–3.3) | 3.1 (2.9–3.3) | 2.7 (1.9–3.0) | 0.11 |
| Birth weight percentile (n = 54) ∗ | 31 (14–54) | 30 (11–54) | 34 (21–54) | 16 (11–80) | 0.47 |
| APGAR score ≥8 at 5 minutes | 94% | 96% | 95% | 86% | 0.55 |
| Cardiac complications † | 7 (13%) | 4 (14%) | 1 (5%) | 2 (29%) | 0.21 |
| Heart failure | 5 (9%) | 2 (7%) | 1 (5%) | 2 (29%) | 0.20 |
| Supraventricular arrhythmia | 4 (7%) | 1 (4%) | 1 (5%) | 2 (29%) | 0.09 |
| Persistent New York Heart Association deterioration | 3 (5%) | 1 (4%) | 1 (5%) | 1 (14%) | 0.71 |
| Valve thrombosis | 1 (2%) | 0 | 0 | 1 (14%) | 0.13 |
| Obstetric complications † | 21 (38%) | 11 (39%) | 6 (30%) | 4 (57%) | 0.50 |
| Hypertension-related disorders | 4 (7%) | 0 | 4 (20%) | 0 | 0.02 |
| Pregnancy-induced hypertension | 2 (4%) | 0 | 2 (10%) | 0 | 0.14 |
| Preeclampsia | 2 (4%) | 0 | 2 (10%) | 0 | 0.14 |
| Premature labor | 4 (7%) | 3 (11%) | 0 | 1 (14%) | 0.33 |
| Preterm premature rupture of membranes | 3 (5%) | 3 (11%) | 0 | 0 | 0.26 |
| Placental abruption | 1 (2%) | 1 (4%) | 0 | 0 | 1.00 |
| Preterm delivery | 13 (24%) | 8 (29%) | 2 (10%) | 3 (43%) | 0.14 |
| Spontaneous | 5 (9%) | 4 (14%) | 0 | 1 (14%) | 0.24 |
| Cardiac maternal indication | 5 (9%) | 2 (7%) | 1 (5%) | 2 (29%) | 0.20 |
| Obstetric indication | 3 (5%) | 2 (7%) | 1 (5%) | 0 | 1.00 |
| Small-for-gestational age | 8 (15%) | 5 (18%) | 3 (15%) | 0 | 0.67 |
| Fetal death | 1 (2%) | 0 | 0 | 1 (14%) | 0.13 |
| Postpartum hemorrhage | 2 (4%) | 2 (7%) | 0 | 0 | 0.36 |
| Postpartum blood loss (ml) | 300 (200–425) | 300 (200–650) | 350 (300–400) | 200 (200–500) | 0.48 |
| Neonatal death | 1 (2%) | 1 (4%) | 0 | 0 | 1.00 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


