Chapter 14 Pregnancy, neonates and children
 Hormonal changes of pregnancy stimulate breathing, causing an increase in tidal volume and hypocapnia.
Hormonal changes of pregnancy stimulate breathing, causing an increase in tidal volume and hypocapnia. Human lung development is incomplete at birth with new alveoli continuing to form until around 3 years of age.
Human lung development is incomplete at birth with new alveoli continuing to form until around 3 years of age. Compared with adults, the respiratory system of a neonate has a very low compliance and a high resistance.
Compared with adults, the respiratory system of a neonate has a very low compliance and a high resistance.Respiratory Function in Pregnancy1
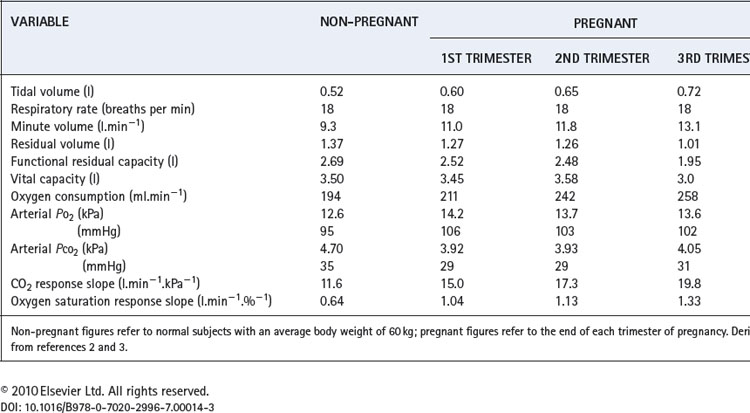
Several physiological changes occur during pregnancy that affect respiratory function. Fluid retention resulting from increasing oestrogen levels causes oedema throughout the airway mucosa, and increases blood volume, substantially increasing oxygen delivery. Progesterone levels rise six-fold through pregnancy and have significant effects on the control of respiration and therefore arterial blood gases. Finally, in the last trimester of pregnancy, the enlarging uterus has a direct impact on respiratory mechanics. A summary of the changes for common respiratory measurements is shown in Table 14.1.
Lung volumes. During the last third of pregnancy the diaphragm becomes displaced cephalad by the expansion of the uterus into the abdomen. This reduces both the residual volume (by about 20%) and expiratory reserve volume, such that functional residual capacity is greatly reduced (Table 14.1). This is particularly true in the supine position, and effectively removes one of the largest stores of oxygen available to the body, making pregnant women very susceptible to hypoxia during anaesthesia or with respiratory disease.
Oxygen consumption. Oxygen consumption increases throughout pregnancy peaking at between 15% and 30% above normal at full term.2,4 The increase is mainly attributable to the demands of the fetus, uterus and placenta, such that when oxygen consumption is expressed per kg of body weight there is little change.
Ventilation. Respiratory rate remains unchanged whilst tidal volume, and therefore minute volume of ventilation, increase by up to 40% above normal at full term. The increase in ventilation is beyond the requirements of the enhanced oxygen uptake or carbon dioxide production so alveolar and arterial Pco2 are reduced to about 4 kPa (30 mmHg).3 This must facilitate clearance of carbon dioxide by the fetus. There is also an increase in alveolar and arterial Po2 of about 1 kPa (7.5 mmHg).
The hyperventilation is attributable to progesterone, and the mechanism is assumed to be a sensitisation of the central chemoreceptors. Pregnancy gives rise to a three-fold increase in the slope of a Pco2/ventilation response curve.2 The hypoxic ventilatory response is increased two-fold, most of the change occurring before the mid-point of gestation, at which time oxygen consumption has hardly begun to increase.5
Dyspnoea occurs in more than half of pregnant women, often beginning early in pregnancy, before the mass effect of the uterus becomes apparent. Dyspnoeic pregnant women, compared with non-dyspnoeic controls, show a greater degree of hyperventilation in spite of having similar plasma progesterone levels.2 Dyspnoea early in pregnancy therefore seems to arise from a greater sensitivity of the chemoreceptors to the increase in progesterone levels. In the third trimester, when dyspnoea on mild exercise is almost universal, the extra effort required by the respiratory muscles to increase tidal volume is believed to be responsible for breathlessness rather than an altered perception of respiratory discomfort.6
The Lungs Before Birth
Embryology
The lungs develop in four stages, under the control of a host of transcriptional factors:7–11
The lungs begin to contain surfactant and are first capable of function by approximately 24–26 weeks, this being a major factor in the viability of premature infants.
Lung Liquid
Fetal lungs contain ‘lung liquid’ (LL) which is secreted by the pulmonary epithelial cells and flows out through the developing airway into the amniotic fluid or gastrointestinal tract, flushing debris from the airways as it does so. A more important function of LL seems to be to prevent the developing lung tissues from collapsing. It is thought that LL maintains the lung at a slight positive pressure relative to the amniotic fluid, and that this expansion is responsible for stimulating cell division and lung growth, particularly with respect to airway branching.10 The respiratory tract in late pregnancy contains some 40 ml of LL, but its turnover is rapid, believed to be of the order of 500 ml per day. Its volume corresponds approximately with the functional residual capacity (FRC) after breathing is established.9
Fetal breathing movements also contribute to lung development. In humans they begin in the middle trimester of pregnancy, and are present for over 20 minutes per hour in the last trimester,13 normally during periods of general fetal activity. During episodes of breathing, the frequency is about 45 breaths per minute and the diaphragm seems to be the main muscle concerned, producing an estimated fluid shift of about 2 ml at each ‘breath’.
Maintenance of a positive pressure in the developing lung requires the upper airway to offer some resistance to the outflow of LL.9 During apnoea, elastic recoil of the lung tissue and continuous production of LL are both opposed by intrinsic laryngeal resistance and a collapsed pharynx. Fetal inspiratory activity, as in the adult, includes dilation of the upper airway. With quiet breathing this would allow increased efflux of LL from the airway, but simultaneous diaphragmatic contraction opposes this. During vigorous breathing movements with the mouth open, pharyngeal fluid may be ‘sucked’ into the airway thus contributing to the expansion of the lungs. Thus fetal breathing movements are believed to contribute to maintaining lung expansion, and their abolition is known to impair lung development.9
Lung Development and Lung Function Later in Life
Complications of pregnancy such as maternal hypertension, pre-eclampsia or the use of antibiotics have all been shown to increase the incidence of wheezing in childhood.14 Small abnormalities of lung development in utero or in the early post-natal phase can adversely affect lung function beyond childhood and into adult life.10,15 For example, in genetically susceptible individuals maternal smoking causes irreversible abnormalities of airway calibre. Abnormalities of amniotic fluid turnover may impair normal lung development in utero, and premature birth alters the normal process of alveolar formation with long term impairment in lung function (see below).
The Fetal Circulation
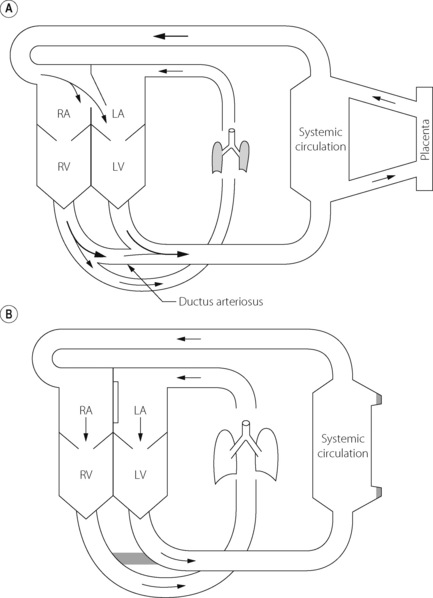
The fetal circulation differs radically from the postnatal circulation (Figure 14.1). Blood from the right heart is deflected away from the lungs, partly through the foramen ovale and partly through the ductus arteriosus. Less than 10% of the output of the right ventricle reaches the lungs, the remainder passing to the systemic circulation and the placenta. Right atrial pressure exceeds left atrial pressure and this maintains the patency of the foramen ovale. Furthermore, because the vascular resistance of the pulmonary circulation exceeds that of the systemic circulation before birth, pressure in the right ventricle exceeds that in the left ventricle and these factors control the direction of flow through the ductus arteriosus.
The umbilical veins drain via the ductus venosus into the inferior vena cava, which therefore contains better oxygenated blood than the superior vena cava. The anatomy of the atria and the foramen ovale is such that the better oxygenated blood from the inferior vena cava passes preferentially into the left atrium and thence to the left ventricle and so to the brain. (This is not shown in Figure 14.1). Overall gas tensions in the fetus are of the order of 6.4 kPa (48 mmHg) for Pco2 and 4 kPa (30 mmHg) for Po2. The fact that the fetus remains apnoeic for much of the time in utero with these blood-gas levels is probably in part attributable to central hypoxic ventilatory depression (page 74).
Events at Birth
Oxygen stores in the fetus are small and it is therefore essential that air breathing and oxygen uptake be established within a few minutes of birth. This requires radical changes in the function of both lungs and circulation.
Factors in the Initiation of Breathing
Fate of the fetal lung liquid. The volume of LL decreases just before and during labour. Some of the residual fluid may be expressed during a vaginal delivery but this is not thought to be a major factor. During in-utero life, the pulmonary epithelium actively secretes lung liquid but at birth this process reverses and the epithelial cells switch to absorption of fluid from the airway.16 Absorption of fluid from airways and alveoli is an active process facilitated by a sodium channel (page 421
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree