Pregnancy and Heart Disease
Approximately 2% of pregnancies are complicated by maternal heart disease. In North America, congenital heart disease is the most common underlying cause, with a declining incidence of rheumatic heart disease. Over recent decades, substantial advances in the care of patients with congenital heart diseases have occurred, with the majority of babies born with heart defects now surviving into their reproductive years. In most cases, the presence of heart disease does not preclude pregnancy, but careful and individualized management is essential in achieving optimal maternal and fetal outcomes. When treating this patient population, the safety of the mother should be the first priority, and drugs, investigations, and interventions should be limited to absolute necessity. Whenever possible, a thorough and informed discussion of the risks of pregnancy and potential risk-minimizing strategies should occur prior to conception. It is also recommended that the care of these patients before, during, and after pregnancy should be delivered by an experienced multidisciplinary team including obstetricians, cardiologists, anesthesiologists, pediatricians, primary care providers, and genetic counselors where appropriate. Cardiac disease may become evident for the first time during pregnancy; conversely many signs and symptoms of normal pregnancy can be suggestive of cardiac pathology.
NORMAL PHYSIOLOGIC CHANGES DURING PREGNANCY
Demands on the cardiovascular system increase steadily during pregnancy, labor, and delivery, and in the postpartum period. Remarkable maternal adaptations to pregnancy occur with major changes in hemodynamics, respiratory parameters, and glucose metabolism (Table 55.1). Because these changes peak late in the second trimester of pregnancy, hemodynamic deterioration in diseased or structurally abnormal hearts most often clinically manifests at this time.
TABLE
55.1 Normal Changes in Hemodynamic, Respiratory, and Metabolic Parameters during Pregnancy
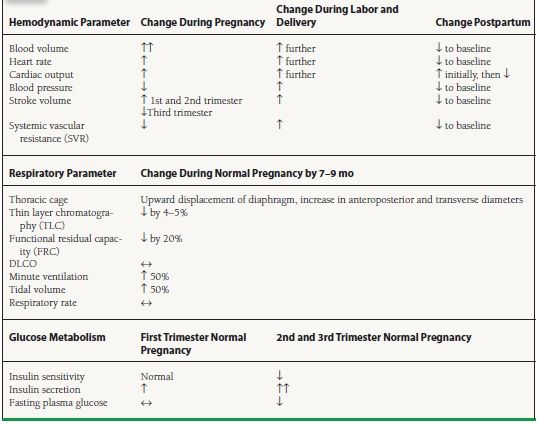
During normal pregnancy, plasma volume increases 40% to 50%, in part due to estrogen-mediated activation of the renin–aldosterone axis. Because red blood cell mass increases 20% to 30%, hemodilution contributes to an overall fall in hemoglobin concentration. Cardiac output rises 30% to 50% above baseline, peaking by the 20th week of gestation and remaining at a plateau until delivery. The change in cardiac output is mediated by (a) increased preload due to the rise in blood volume, (b) reduced afterload due to a fall in systemic vascular resistance (SVR), and (c) a rise in the maternal heart rate by 10 to 15 beats/min (bpm). Stroke volume begins to rise by 5 weeks’ gestation and peaks by 31 weeks. In the third trimester, caval compression by the gravid uterus causes stroke volume to fall slightly. However, a compensatory rise in heart rate allows maintenance of cardiac output. The direct effect of pregnancy on cardiac contractility is controversial. Blood pressure typically falls to 10 mm Hg below baseline by the end of the second trimester, due to decreased SVR induced by hormonal changes and by the addition of low-resistance vessels in the uteroplacental bed. Redistribution of cardiac output is facilitated by uterine vasodilatation, with an increase from 100 mL/min uterine blood flow in the nonpregnant state to approximately 1,200 mL/min at term. Maternal oxygen consumption, which includes the oxygen demands of the developing fetus, peaks at 30% above the nonpregnant level. These hemodynamic changes are better tolerated by volume overload lesions than by fixed obstructions to output.
During labor and delivery, dramatic variations in hemodynamic status add further stress to the cardiovascular system. Each uterine contraction displaces 300 to 500 mL of blood into the general circulation. In combination with elevated heart rates, cardiac output during contractions can increase up to 75% over baseline during labor, with typical values around 9 L/min. Use of epidural anesthesia slightly reduces cardiac output due to peripheral vasodilatation, and general anesthetic may lower output more substantially. Mean systemic pressure usually rises during labor with maternal pain and anxiety. Blood loss during delivery (averaging 300 to 400 mL for vaginal delivery and 500 to 800 mL for cesarean section) can further compromise hemodynamics.
During the postpartum period, cardiovascular hemodynamics is altered once again by the relief of vena caval compression. The increase in venous return augments preload and cardiac output, resulting in an increase in renal blood flow and a brisk diuresis. Cardiovascular homeostasis is largely restored to the prepregnant baseline within 3 to 4 weeks following delivery, although it is suggested that a slight augmentation of cardiac output may persist for as long as 12 months.
The hemodynamic response to exercise is altered in pregnancy In the sitting position, any given level of exertion results in a greater cardiac output than the nonpregnant state, and maximal output is attained at lower levels of activity Animal models show a decrease in uterine blood flow with exercise, and regular aerobic endurance exercise in humans during pregnancy has been shown to reduce birth weight. The long-term implications of this observation are unclear, although they are likely greater for mothers with heart disease than those without. Activity recommendations for pregnant women with heart disease should be based on the level of symptoms.
Pregnancy induces marked changes in respiratory parameters. The gravid uterus gradually limits diaphragmatic excursion, resulting in reductions in total lung capacity and functional residual capacity. These changes are countered by hormonally induced increases in airway dilatation. In addition, minute ventilation increases 45% via an increase in tidal volume.
Pregnancy is also characterized by a complex series of hormonal and metabolic changes that govern glucose regulation. Typically, a state of maternal insulin resistance develops during the second and third trimesters. Insulin resistance is a physiologic response that favors a shift in the glucose supply to the fetus. In normal women, however, insulin resistance is countered by a steady increase in basal insulin secretion and a marked increase in insulin secretion immediately after a glucose load (first phase). In contrast, women with gestational diabetes exhibit impaired pancreatic β-cell secretory function and demonstrate a blunted first-phase insulin secretion response to glucose loading. The cardiovascular consequences of gestational diabetes can include macrosomia, shoulder dystocia, future development of maternal type 2 diabetes, and an increased risk of obesity and type 2 diabetes in the offspring.
Fatigue, dyspnea, and decreased exercise capacity are common in normal pregnancies and can mimic cardiac disease. Pregnant women usually have peripheral edema, lateral displacement of the point of maximum impulse, and a brisk and full carotid upstroke. Most pregnant women have audible physiologic systolic murmurs, created by augmented blood flow. This systolic murmur is typically located on the left sternal border, is wide-peaking, and is associated with exaggerated physiologic splitting of S2. A physiologic third heart sound (S3) can often be appreciated, as can a continuous venous hum or mammary soufflé. Symptoms that are unusual during normal pregnancy and may signal true cardiac pathology include chest pain, orthopnea, or paroxysmal nocturnal dyspnea. A fourth heart sound, a loud systolic murmur, a purely diastolic murmur, fixed splitting of S2, or pulmonary edema, are not expected findings and should be promptly investigated.
ASSESSMENT OF RISK IN PATIENTS WITH PREEXISTING CARDIAC DISEASE
Planning for Optimal Maternal and Fetal Outcomes
It is advised that individuals with structural cardiac disease who have undergone surgical or catheter-based repair should not be considered “corrected,” as some residual disease almost always remains and the responses to the physiology of pregnancy can be unpredictable. Whenever possible, women with known preexisting cardiac lesions should receive preconception counseling. This should include contraceptive advice, quantification of maternal and fetal risks during pregnancy, and discussion of possible long-term morbidity and mortality after pregnancy. Unfortunately, many women with preexisting heart disease do not appear to be aware of the risks of pregnancy. In one questionnaire-based study of 116 adult females with congenital heart disease, of which 55% had been pregnant at least once, 37% of respondents reported that they had never been informed that they were at increased risk for maternal cardiac complications during pregnancy. The following conditions are generally considered contraindications to pregnancy: severe pulmonary hypertension of any etiology, severe fixed obstructive cardiac lesions, heart failure New York Heart Association (NYHA) class III-IV, left ventricular ejection fraction (LVEF) <40%, prior peripartum cardiomyopathy (PPCM), dilated unstable aorta of 40 to 45 mm or above, or severe cyanosis. The cardiac disease in pregnancy (CARPREG) risk score is composed of four clinical features found to be predictive of maternal cardiac complications (Table 55.2). Each risk factor was assigned a value of one point. The maternal cardiac event rate associated with 0, 1, and >1 points was 5%, 27%, and 75%, respectively. The more recent ZAHARA predictors were developed in a population of 1,802 congenital heart disease patients. Although such scores serve as an overall assessment of risk, prepregnancy counseling should be tailored according to specific cardiac lesions. In the following section, congenital and acquired cardiac lesions are classified as low, intermediate, or high risk (Table 55.3).
TABLE
55.2 Predictors of Maternal Risk for Cardiac Complications
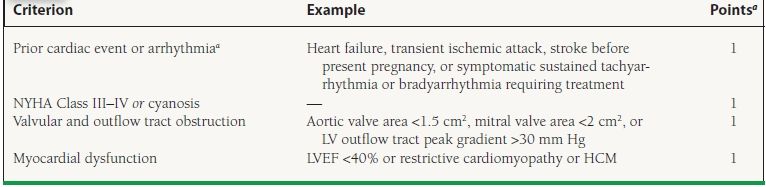
aMaternal cardiac event rate for 0, 1, and >1 point is 5%, 27% and 75%, respectively.
LVEF, left ventricular ejection fraction.
Adapted from Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521, with permission.
TABLE
55.3 Maternal Cardiac Lesions and Risk of Cardiac Complications during Pregnancy
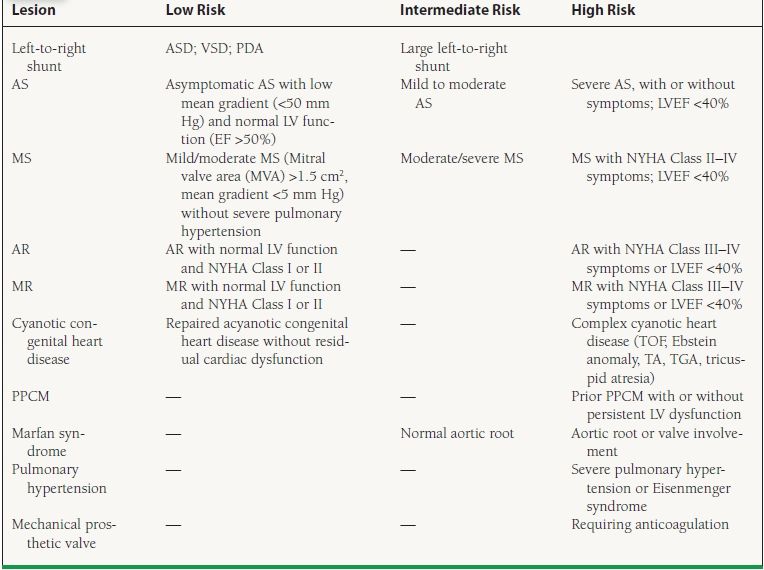
AS, aortic stenosis; LV left ventricle; EF, ejection fraction; AR, aortic regurgitation; NYHA, New York Heart Association; MVP, mitral valve prolapse; MS, mitral stenosis; MVA, mitral valve area; PS, pulmonary stenosis; TOF, tetralogy of Fallot; TA, truncus arteriosus; TGA, transposition of the great arteries; PPCM, peripartum cardiomyopathy.
The likelihood of cardiac disease in the developing fetus is dependent upon the exact nature of the mother’s condition, but overall 5% of women with cardiac disease themselves have offspring with a congenital heart condition. A fetal anomaly scan is routinely offered around 20 to 26 weeks, which screens for cardiac defects.
Vaginal delivery is optimal for most expectant mothers with heart disease. Left lateral decubital positioning helps to maintain venous return. The second stage of labor should be assisted if necessary with forceps or vacuum extraction in order to avoid a long labor. Indications for cesarean section can include obstetric indications, warfarin anticoagulation (due to the risk of neonatal intracranial hemorrhage during delivery), severe maternal pulmonary hypertension, severe fixed obstructive cardiac lesions, or an unstable aorta. At the 24th week of gestation or beyond, a life-threatening cardiac complication should prompt consideration of steroids for fetal lung maturity and emergent cesarean section delivery. Successful delivery does not resolve the maternal risk; even in women unaffected by cardiac disease, a significant proportion of pregnancy-related deaths occur in the first week postpartum.
The 2007 American Heart Association (AHA) guidelines on prevention of infective endocarditis provided revised recommendations for the conditions and procedures with which antibiotic prophylaxis should be administered. Cardiac conditions associated with the highest risk of adverse outcomes from endocarditis are stated as prosthetic valves, previous infective endocarditis, unrepaired or incompletely repaired congenital heart disease, congenital defects repaired with prosthetic material for the first 6 months post repair, and cardiac transplant recipients who develop valvulopathy. Patients meeting these criteria are still recommended to receive prophylaxis with dental procedures; however, the guidelines state that antibiotics solely to prevent endocarditis are not recommended for genitourinary (GU) or gastrointestinal (GI) tract procedures. Vaginal delivery is not specifically mentioned within these guidelines. The 2008 American College of Cardiology (ACC)/AHA guidelines for the management of adults with congenital heart disease state that it is reasonable to consider antibiotic prophylaxis before vaginal delivery at the time of membrane rupture in the patients within this high-risk patient group. In clinical practice, physicians should discuss the recommendations and pathophysiology with expectant mothers who meet these high-risk criteria early in pregnancy, and jointly form an individualized plan regarding antibiotic prophylaxis for vaginal delivery.
Specific Congenital or Acquired Cardiac Lesions
Low-Risk Lesions
Atrial Septal Defect Ostium secundum atrial septal defect (ASD), the most common congenital cardiac lesion encountered during pregnancy, is usually well tolerated. An uncorrected ASD does carry a small increased risk of paradoxical embolism and so deep vein thrombosis (DVT) prevention should be meticulous. With advancing maternal age (especially >40 years), uncomplicated ASD may be accompanied by a higher incidence of supraventricular arrhythmias (e.g., atrial fibrillation or atrial flutter). Although it is unusual for secundum ASD to cause pulmonary hypertension during the childbearing years, the presence of pulmonary hypertension substantially increases the risk of cardiac complications during pregnancy. A secundum ASD that has been repaired prior to pregnancy is not associated with any increased risk of cardiac complications. Device or operative repair of an ASD prior to conception is preferable. It has recently been highlighted that individuals with simple secundum ASDs appear to be at elevated risk of bacterial endocarditis. Holt–Oram syndrome, a rare heart-upper limb malformation complex that includes ASD, requires additional consideration given the potential for autosomal dominant transmission of the TBX5 gene defect to offspring.
Ventricular Septal Defect Isolated ventricular septal defect (VSD) is also a low-risk lesion that is usually well tolerated during pregnancy. However, VSD accompanied by pulmonary hypertension and/or Eisenmenger syndrome carries a high risk for cardiac complications. VSD can occur in conjunction with other congenital cardiac lesions, including ASD, patent ductus arteriosus (PDA), mitral regurgitation (MR), and transposition of the great arteries. The risk associated with a VSD that was repaired prior to the development of pulmonary hypertension is negligible.
Patent Ductus Arteriosus
The presence of a PDA during pregnancy is not associated with additional maternal risk, provided that the shunt is small to moderate and that the pulmonary artery pressures are normal. Percutaneous closure is now first-line, and it is considered reasonable to close even asymptomatic small PDAs. Following repair of more significant PDAs, women are at no additional risk for complications during pregnancy.
Mitral Valve Prolapse In isolation, mitral valve prolapse (MVP) rarely causes any difficulties during pregnancy. MVP is specifically stated as a low risk condition for endocarditis in the AHA 2007 guidelines, and is not an indication for antibiotic prophylaxis at delivery.
Mitral Regurgitation Chronic regurgitant lesions are generally well tolerated during pregnancy. In chronic MR, the physiologic reduction in systemic vascular resistance (SVR) partially compensates for the additional volume overload generated by the regurgitant valve. However, the development of new atrial fibrillation or severe hypertension can disrupt this balance and precipitate hemodynamic deterioration. In contrast, acute MR (e.g., from rupture of chordae tendineae) is not well tolerated and can produce flash pulmonary edema and/or life-threatening cardiac decompensation. The most common causes of MR are rheumatic heart disease and myxomatous degeneration. Hypertrophic cardiomyopathy (HCM) and mitral annular dilatation secondary to dilated cardiomyopathy can also result in MR.
Women with preexisting severe MR may develop heart failure symptoms during pregnancy, especially during the third trimester. In general, these symptoms can be managed medically with judicious use of diuretics and afterload-reducing agents. Nitrates, hydralazine, and dihydropyridine calcium channel-blocking agents can serve as relatively safe afterload-reducing agents in pregnant women. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) are strictly contraindicated during pregnancy. Women with severe symptomatic MR prior to pregnancy may consider operative repair prior to conception. Although repair is strongly preferred to valve replacement before pregnancy, the success of operative repair is dependent on suitable valve anatomy.
Aortic Regurgitation Like chronic MR, chronic aortic regurgitation (AR) is also generally well tolerated during pregnancy. In addition to the physiologic fall in SVR, the tachycardia of pregnancy shortens diastole and reduces the aortic regurgitant fraction. Marfan syndrome should be considered as an etiology, because of the implications regarding aortic root stability during pregnancy. AR is generally well tolerated and is usually medically managed during pregnancy with diuretics and afterload reducers. Operative repair prior to pregnancy is feasible in certain patients, especially when the valve is anatomically bicuspid. However, the long-term durability of repair may not be superior to valve replacement. During pregnancy, surgical intervention for both mitral and AR is usually performed only for refractory heart failure, which is a rare occurrence.
Pulmonary Stenosis As an isolated lesion, pulmonic stenosis is well tolerated during pregnancy. Severe, symptomatic pulmonary stenosis (PS) may be treated with percutaneous pulmonary valvuloplasty prior to conception. If necessary during pregnancy, percutaneous pulmonary valvuloplasty should be delayed until after the first trimester to avoid fetal radiation exposure during early development. PS frequently coexists with other congenital cardiac lesions that may cause cyanotic heart disease.
Moderate-Risk Lesions
Mitral Stenosis Mitral stenosis (MS) in women of childbearing age is most often rheumatic in origin. It is the most common valve disease complicating pregnancy worldwide and one of the most poorly tolerated. Symptoms occur in up to a quarter of affected patients, and usually become apparent by the 20th week. Patients with a mitral valve area <1.5 cm2 face a substantial risk of heart failure, cardiac arrhythmia, and/or intrauterine growth retardation during pregnancy. Increased blood volume and heart rate during pregnancy lead to left atrial pressure elevation, which can result in pulmonary edema. Additional displacement of blood volume into the systemic circulation during uterine contractions makes labor particularly hazardous.
MS requires close and regular follow-up during pregnancy. Echocardiography should be performed at the end of the first and second trimesters, and monthly during the third trimester. Beta-blockers and diuretics should be used to control signs and symptoms of pulmonary congestion and to maintain the estimated pulmonary artery pressure below 50 mm Hg.
Although mild MS can often be managed with conservative medical therapy during pregnancy, patients with moderate to severe MS should consider correction prior to conception. When pregnancy has already occurred and medical therapy is insufficient to control severe symptomatic MS, intervention during pregnancy may be necessary. Percutaneous mitral balloon valvuloplasty (PMBV) is the therapeutic option of choice. Its safety and feasibility during pregnancy have been well established. Radiation exposure to the fetus is minimized by abdominal lead shielding, use of transesophageal echocardiographic guidance, and omission of invasive hemodynamic measurements and angiography. When PMBV cannot be performed, open surgical commissurotomy is the preferred surgical correction. Although this procedure is considered safe for the mother, it carries a 2% to 12% risk of fetal mortality. Cardiopulmonary bypass during pregnancy should be performed with normothermic perfusion and high flow volumes, to reduce fetal morbidity and mortality. Women past 20 weeks’ gestation should be positioned in the lateral decubitus position during surgery, to avoid uterine compression of the inferior vena cava. If possible, hyperkalemic arrest should be avoided, because of the potential for hyperkalemic solutions to reach the fetal circulation. In the current era it appears that maternal mortality with cardiopulmonary bypass is equivalent to that expected from a nonpregnant female, unless the operation is emergent.
The combination of atrial fibrillation and MS in the pregnant patient may result in a rapid rise in left atrial pressure and acute pulmonary edema. Treatment consists of heart rate control with digoxin and beta-blockers plus gentle reduction of blood volume and left atrial pressure with diuretics. Hemodynamic deterioration is an indication for electrocardioversion, which can be performed safely during pregnancy. The development of atrial fibrillation is an indication for the initiation of anticoagulation, which is discussed in greater detail in the section, “Anticoagulation during Pregnancy.”
Most patients with MS can undergo vaginal delivery. However, Swan–Ganz hemodynamic monitoring during labor, delivery, and for several hours into the postpartum period is advisable in patients with symptoms of heart failure or with moderate to severe MS. In these patients, epidural anesthesia during labor and delivery is usually better tolerated than general anesthesia.
Aortic Stenosis The most common etiology of aortic stenosis (AS) in women of childbearing age is a congenitally bicuspid valve. Other, less common etiologies include rheumatic heart disease, calcific valvular disease, and a unicuspid aortic valve. Mild to moderate AS with preserved left ventricular (LV) function usually is well tolerated during pregnancy. However severe AS (aortic valve area <1.0 cm2, mean gradient >50 mm Hg) significantly increases the maternal and fetal risks of pregnancy and women with uncorrected severe AS should be counseled against conception. Classic symptoms of AS such as dyspnea, angina pectoris, or syncope usually become apparent late in the second or early third trimester.
Ideally, women with known severe AS should undergo valve correction prior to conception. Although percutaneous aortic balloon valvuloplasty (PABV) prior to pregnancy can decrease the risk of pregnancy, labor, and delivery, it has limited durability and is unlikely to relieve AS in the long term. Therefore, surgical correction is the preferred approach.
Bioprosthetic valves have limited durability; young patients will likely require reoperation within 10 to 15 years. However, implantation of a bioprosthetic valve avoids the need for anticoagulation during pregnancy. Mechanical valves have greater durability but require anticoagulation, which independently increases both the maternal and fetal complication risk during pregnancy. The decision to implant a bioprosthetic versus mechanical valve is complex and should be made in consultation with both a cardiologist and a cardiothoracic surgeon.
Management of mild to moderate AS during pregnancy is largely conservative. When severe symptomatic AS is diagnosed during pregnancy, PABV should be performed prior to the demands of labor and delivery. Aortic insufficiency that occurs as a postprocedural complication of PABV usually is well tolerated during labor and delivery. Vaginal or assisted vaginal delivery is usually well tolerated, although spinal and epidural anesthesia are discouraged during labor and delivery because of their vasodilatory effects. Invasive hemodynamic monitoring and is recommended during labor and delivery.
Coarctation of the Aorta Coarctation of the aorta is a narrowing in the region of the ligamentum arteriosum, just distal to the origin of the left subclavian artery usually presenting with resistant hypertension in childhood. Coarctation is well tolerated during pregnancy, although hypertension, heart failure, angina, and aortic dissection are possible complications. Coarctation can be associated with intracerebral aneurysms, which may rupture during pregnancy. Hypotension in vascular beds distal to the coarctation can compromise uteroplacental blood flow, resulting in intrauterine growth retardation. Coarctation of the aorta is often associated with a congenitally bicuspid aortic valve, which increases the risk of infective endocarditis.
If at all possible, coarctation of the aorta should be corrected prior to pregnancy with standard surgical repair or balloon angioplasty with endovascular stent placement. Correction of coarctation during pregnancy is indicated in patients with severe uncontrollable hypertension, heart failure, or uterine hypoperfusion. The aortic wall adjacent to an area of coarctation has histologic features of cystic medial necrosis, which renders it vulnerable to dissection. Thus, women who underwent surgical repair of an aortic coarctation in childhood remain at risk for complications during pregnancy.
Marfan Syndrome Marfan syndrome is a connective tissue disorder resulting from mutations in the fibrillin gene that are inherited in an autosomal dominant fashion (i.e., 50% of offspring will inherit the disorder, regardless of gender). Thus, women with Marfan syndrome should receive genetic counseling well in advance of pregnancy consideration. The clinical manifestations of Marfan syndrome include skeletal abnormalities, ectopia lentis, and cardiovascular abnormalities, such as aortic root dilatation with or without AR, aortic dissection, and MVP. However, Marfan syndrome is a heterogeneous disorder with highly variable disease penetrance.
It is estimated that pregnancy in patients with Marfan syndrome carries a 1% risk of serious cardiac complications; this risk rises with increasing aortic root dimensions. Women with Marfan syndrome are more vulnerable to aortic dissection and/or rupture during pregnancy because of the additional weakness in the aortic wall imposed by hormonal changes. Interestingly, the occurrence of dissection appears to peak at 3 to 20 days postpartum, with a hypothesis being that oxytocin stimulation during breast-feeding may activate the extracellular signal-regulated kinase (ERK) pathway, which has been recently implicated in the pathophysiology of this condition. In addition, women with Marfan syndrome may be more prone to spontaneous abortion and preterm labor.
Screening echocardiography should be performed prior to pregnancy. Enlargement of the aortic root >4.5 cm is associated with maternal mortality as high as 10%. There is some evidence that pregnancy in women with Marfan syndrome may be relatively safe up to 4.5 cm, and therefore in women with an aortic diameter of 4.0 to 4.5 cm pertinent risk factors for dissection (family history of dissection, rapid growth) should be taken into account, and body surface area factored in too. The 2011 European Society of Cardiology guidelines strongly recommend preconception elective aortic root repair above 4.5 cm and support individualization of management in the range 4.0 to 4.5 cm; the 2010 ACC/AHA guidelines on thoracic aortic disease state that it is “reasonable” to prophylactically replace the aortic root and ascending aorta when the diameter exceeds 4.0 cm. The aortic root should be monitored by serial echocardiography throughout pregnancy, with progressive dilatation warranting termination of pregnancy and/or timely aortic repair or replacement.
Medical management involves the use of beta-blockers throughout pregnancy to reduce the risk of aortic rupture, careful control of blood pressure, and consideration of general anesthesia and cesarean section at the time of delivery to maximize hemodynamic control. Women with Marfan syndrome who do not manifest any cardiac abnormalities have a low rate of complications and can usually tolerate a normal vaginal delivery. Spinal or epidural anesthesia is advised, to minimize the pain and stress of labor.
High-Risk Lesions
Eisenmenger Syndrome The Eisenmenger syndrome is a consequence of uncorrected long-standing left-to-right shunts. Over time, pulmonary artery pressures approach and can exceed systemic pressures, resulting in reversal of the shunt flow direction from right to left and cyanosis. Eisenmenger syndrome is a possible common endpoint of multiple congenital lesions, including ASD, VSD, and PDA. Maternal mortality in women with Eisenmenger syndrome ranges from 30% to 50%, with a 50% risk of fetal loss if the mother survives. Mortality is frequently caused by complications of thromboembolic disease. Decompensation occurs most frequently during the first week after delivery. Fetal risk due to maternal hypoxemia is substantial, with a high incidence of fetal loss, premature delivery, intrauterine growth retardation, and perinatal death.
Because of the considerable risk to both the mother and the fetus, pregnancy is not advised for women with Eisenmenger syndrome. If pregnancy should occur, therapeutic abortion is recommended. Women who choose to continue with pregnancy are advised to restrict physical activity, use continuous oxygen for at least the third trimester and consider use of pulmonary vasodilating drugs such as iloprost and prostacyclin. Anticoagulation is recommended during the third trimester and for 4 weeks after delivery. The most vulnerable period for the mother is labor and delivery and the first week postpartum. Vaginal delivery, facilitated by vacuum or low forceps extraction, is the delivery method of choice. Cesarean delivery is associated with a substantially higher mortality than the vaginal route. Anesthetic management includes central venous and arterial pressure monitoring, with maintenance of adequate SVR and intravenous volume and prevention of sudden increases in pulmonary vascular resistance (PVR).
Complex Cyanotic Congenital Heart Disease More women born with cyanotic congenital heart disease are surviving to childbearing age. In general, pregnancy is not recommended for women with uncorrected lesions. A low maternal oxygen saturation (<85%) correlated with a very low rate of live-born infants (12%) in one study of pregnancy in cyanotic heart disease. The most common cyanotic congenital defect is tetralogy of Fallot, which is characterized by a VSD, pulmonic stenosis, right ventricular outflow tract obstruction, and an overriding aorta. Women with tetralogy of Fallot who have undergone successful repair during childhood may tolerate pregnancy well, provided that they have little or no residual right ventricular outflow tract gradient, no pulmonary hypertension, and preserved ventricular function. Genetic counseling and screening for the 22q11 deletion should be offered as its transmission is autosomal dominant. Ebstein anomaly, characterized by abnormal right ventricular function, apical displacement of the tricuspid valve septal leaflet, and tricuspid regurgitation, is often associated with the Wolf–Parkinson–White syndrome. Pregnancy can precipitate supraventricular arrhythmias that may be rapidly conducted over the accessory pathway. Surgical correction reduces the maternal risk of pregnancy, but does not reduce the risk of congenital anomalies in the fetus. Individuals with a single functional ventricle will usually have been palliated with a version of the Fontan procedure during childhood. Heart failure, thromboembolism, and atrial arrhythmias occur in 10% to 20% pregnant patients with this anatomy, and fetal loss is up to 50%. Experience during pregnancy in women with surgically corrected D-transposition of the great arteries, truncus arteriosus, or tricuspid atresia is limited. Women with congenitally corrected transposition (L-transposition) and no cyanosis, heart failure, or conduction disease should tolerate pregnancy well. Preterm delivery rates in these complex conditions range from 22% to 65%, and an elevated rate of premature rupture of membranes has been associated with Fontan patients and transposition.
Cardiomyopathy Women with heart failure of any etiology and ejection fraction <40% or NYHA class III-IV symptoms should be counseled to avoid pregnancy. PPCM and its implications for subsequent pregnancies are discussed further in the following section. HCM is associated with increased maternal morbidity and mortality. Although an increase in blood volume helps to reduce intracavitary or LV outflow tract gradients, tachycardia and a reduction in SVR can exacerbate outflow tract obstruction. Avoidance of volume depletion helps to prevent hemodynamic deterioration in these patients. Vaginal delivery is usually well tolerated. Whenever possible, women should receive genetic counseling prior to conception, because the heritability of certain forms of HCM approaches 50%.
CARDIOVASCULAR DISORDERS ACQUIRED DURING OR AFTER PREGNANCY
Peripartum Cardiomyopathy
PPCM is defined as the development of idiopathic LV systolic dysfunction (demonstrated by echocardiography) in the last month of pregnancy or up to the first 5 months postpartum in women without preexisting cardiac dysfunction. The incidence of PPCM in the United States is estimated to be 1 in 3,000 to 1 in 4,000 live births; the incidence appears to be highest in Africa and Haiti (occurring in 1 in 300 pregnancies). Certain risk factors are appreciated: maternal age >30 years, obesity, multiparity, multiple fetuses, history of preeclampsia, eclampsia, or chronic hypertension, African descent, low socioeconomic status, or tocolytic therapy with beta-agonists. There does not appear to be a hereditary predisposition.
PPCM has long been regarded as a disease of unknown etiology, with possible triggers including viral infection and autoimmunity. Recent mouse model research has demonstrated a role for a 16 kDa protein derived from proteolytic cleavage of prolactin under oxidative stress. This derivative is cardiotoxic, antiangiogenic, proaptotic, and proinflammatory and is observed in higher levels in PPCM patients. Furthermore, small numbers of postpartum women with PPCM have shown favorable cardiac outcomes with bromocriptine, which inhibits prolactin secretion via dopamine receptor agonism.
Medical therapy for PPCM is similar to therapy for cardiomyopathies of other etiologies. Digoxin and diuretics may be used safely during pregnancy and while breastfeeding. Beta-blockers are generally considered safe during pregnancy, although there have been case reports of fetal bradycardia and growth retardation. ACE inhibitors and ARBs are strictly contraindicated throughout pregnancy. ACE inhibitor fetopathy includes oligohydramnios, intrauterine growth retardation, hypocalvaria, renal dysplasia, anuria, and death. Hydralazine is an effective afterload-reducing agent, although it is currently listed as a category C agent (adequate and well-controlled studies in pregnant patients are lacking, and it should be used only when the expected benefit outweighs the potential risk to the fetus). Anticoagulation should be considered. When medical therapy is not successful, women with PPCM may ultimately require advanced mechanical support or cardiac transplantation.
The prognosis after development of PPCM is variable. Approximately 50% of women recover completely normal heart size and function, usually within 6 months of delivery. The remainder either experience stable LV dysfunction or continue to experience clinical deterioration. Mortality at 2 years appears to be around 15%. Women with PPCM who attempt a subsequent pregnancy face a high risk of complications, including deterioration of LV function, symptomatic heart failure, and death. Some experts counsel affected women against subsequent pregnancies. However the majority of maternal deaths have occurred in women whose LV function remained abnormal prior to becoming pregnant again, and LVEF at first PPCM diagnosis seems to be a major prognostic indicator. Therefore, an EF <25% at initial presentation, and/or persistence of any degree of EF reduction at the time of consultation, are considered definite contraindications to future pregnancy.
Hypertension in Pregnancy
Hypertension during pregnancy can be classified into three main categories: chronic hypertension, gestational hypertension, and preeclampsia, with or without preexisting hypertension. Hypertensive disorders can complicate 5% to 15% of pregnancies and are a major cause of maternal morbidity and mortality
Chronic, or preexisting, hypertension is defined as blood pressure ≥140/90 mm Hg present prior to pregnancy, before the 20th week of gestation, or persisting beyond the 42nd postpartum day. Drug therapy for diastolic blood pressure ≥110 mm Hg has been shown to reduce the risk of stroke and cardiovascular complications. Options for drug therapy are shown in Table 55.4.
TABLE
55.4 Drug Therapy for Mild to Moderate Chronic Hypertension in Pregnancy
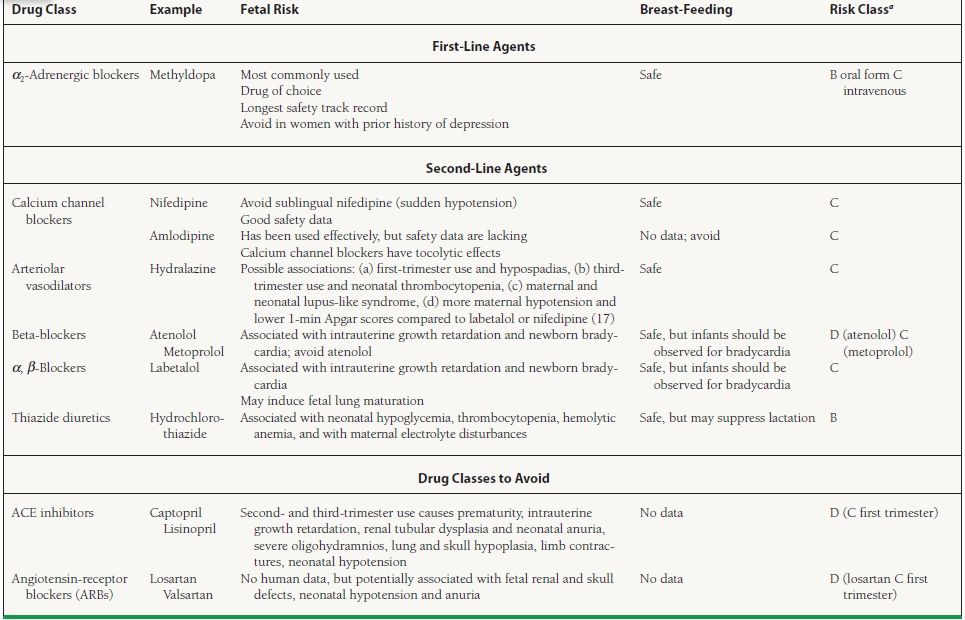
aRisk Class C: Either studies in animals have revealed adverse effects on the fetus and there are no controlled studies in women, or studies in women are not available. Drug should be given only if the potential bei justifies the potential risk to the fetus. Risk Class D: There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk.



