Restrictive mitral annuloplasty is a surgical treatment option for patients with heart failure (HF) and functional mitral regurgitation (MR). However, recurrent MR has been reported at mid-term follow-up. The aim of the present study was to identify the echocardiographic predictors of recurrent MR in patients with HF undergoing mitral annuloplasty. During a mean follow-up of 2.6 ± 1.6 years, 109 patients with HF (49% ischemic and 51% idiopathic dilated cardiomyopathy) who had undergone mitral valve repair were followed up (of 122 total patients). The severity of MR was quantified, and the following parameters were measured before intervention and at the mid-term follow-up examination: left ventricular (LV) and left atrial volumes and dimensions, LV sphericity index, mitral annular area, and mitral valve geometry parameters. At mid-term follow-up, 21 patients presented with significant MR (grade 2 to 4), and 88 patients had only MR grade 0 to 1. Both groups of patients had had a similar preoperative MR grade, mitral annular area, and LV volume and dimension. In contrast, patients with recurrent MR had had increased preoperative posterior and anterior leaflet angles, tenting height, tenting area, and LV sphericity index compared to the patients without recurrent MR. Of the different parameters of mitral and LV geometry, the distal mitral anterior leaflet angle (hazard ratio 1.48, 95% confidence interval 1.32 to 1.66, p <0.001) and posterior leaflet angle (hazard ratio 1.13, 95% confidence interval 1.07 to 1.19, p <0.001) were independent determinants of MR at mid-term follow-up. In conclusion, in patients with HF of ischemic or idiopathic etiology and functional MR, distal mitral leaflet tethering and posterior mitral leaflet tethering were associated with recurrent MR after restrictive mitral annuloplasty.
The mechanism of functional mitral regurgitation (MR) is complex, with mitral annular dilation and tethering of mitral leaflets contributing to the MR pathophysiology. Some of these pathophysiologic issues, either related to left ventricular (LV) geometry or mitral valve geometry itself, could contribute to recurrent MR after mitral annuloplasty. Previous studies focusing on the mechanisms of recurrent MR have been conducted in separate series of patients with ischemic and idiopathic dilated cardiomyopathy. The present study identified the preoperative echocardiographic predictors of mid-term recurrent MR after successful mitral valve annuloplasty in a cohort of patients with heart failure (HF) with idiopathic dilated or ischemic cardiomyopathy.
Methods
A total of 122 patients with HF and moderate to severe MR were included. The patients were scheduled for restrictive mitral annuloplasty, accompanied by coronary artery bypass grafting if indicated. In patients with HF with idiopathic cardiomyopathy, restrictive mitral annuloplasty was performed with concomitant placement of a CorCap (Acorn Cardiovascular, St. Paul, Minnesota) cardiac support device if significant LV dilation (LV diameter >65 mm) was measured on the preoperative echocardiographic examination. All patients underwent surgery using a midline sternotomy with normothermic cardiopulmonary bypass and intermittent anterograde warm blood cardioplegia. The mitral valve was exposed through a transseptal approach. The ring size (Carpentier Edwards Physioring, Edwards Lifesciences, Irving, California) was determined after careful measurement of the height of the anterior leaflet. Then, downsizing by 2 sizes was performed (ie, when ring measured 30, the size of the annuloplasty ring was 26). The rings were inserted using 14 to 16 deep U -shaped simple horizontal sutures with Ethibond 2-0 (Ethicon, Somerville, New Jersey) or Ti-Cron 2-0 (Syneture, Norwalk, Connecticut). Tricuspid annuloplasty was performed with a Carpentier Edwards Classic or MC3 ring (Edwards Lifesciences) in patients with tricuspid regurgitation exceeding grade 2 or in the presence of a dilated tricuspid annulus >40 mm (or 21 mm/m 2 indexed to the body surface area) on the echocardiogram. In all patients, the results of mitral annuloplasty were assessed by intraoperative transoesophagal echocardiography. No residual MR and the coaptation of mitral leaflets of ≥8 mm at the A2-P2 level (A2: middle scallop of the anterior mitral leaflet, P2: middle scallop of the posterior mitral leaflet) were the criteria for successful mitral valve repair. If these criteria were not fulfilled, additional downsizing was performed.
In patients undergoing surgical revascularization, the coronary artery bypass grafts were implanted before mitral valve annuloplasty. If a cardiac support device (CorCap, Acorn Cardiovascular) was used, it was applied on the beating heart, and then it was fixed by sutures to the dorsal base of the heart, along the atrioventricular groove. After completion of the mitral valve repair, the extracorporeal circulation was weaned out and the final fitting of CorCap was ensured on a full and beating heart with appropriate filling. The aim was to obtain a snug fit, without reduction of LV diameter of >10% (as measured by transesophageal echocardiography) compared to preoperative dimensions, as described earlier.
Before surgical intervention, transthoracic echocardiography (System Five or Vivid 7, GE Norway, Horten, Norway) was performed and repeated at hospital discharge and mid-term follow-up (2.6 ± 1.6 years). Transthoracic echocardiography at discharge was used to confirm the absence of MR, and the preoperative and mid-term follow-up echocardiographic examinations were used to perform measurements of LV and left atrial (LA) volumes and dimensions, geometric analysis of the left ventricle and mitral valve, and quantification of MR.
The severity of MR was quantitatively determined by proximal isovelocity surface area and by vena contracta method according to the current guidelines. The effective regurgitant orifice and regurgitant volume were calculated according to the formula. The vena contracta was defined as the narrowest part of the regurgitant jet recorded in the parasternal long-axis view.
The LV and LA volumes and LV geometry were measured according to the recommendations of the American Society of Echocardiography and European Association of Echocardiography for chamber quantification. In the parasternal long-axis view, the LV end-diastolic diameter, LV end-systolic diameter, and LA diameter were measured. The LV end-diastolic volume, LV end-systolic volume, and LV ejection fraction were estimated using Simpson’s disk method from the apical 4- and 2-chamber views. The LA volume was assessed using the area-length method. The systolic and diastolic LV sphericity indexes were calculated as the ratio between the LV short-axis diameter and the LV long-axis diameter at end-systole and end-diastole, respectively.
To evaluate the apical displacement of the mitral leaflets, the leaflet tethering lengths were measured in the apical 4- and 2-chamber views in mid-systole ( Figure 1 ). The end-diastolic and end-systolic frames were determined from the mitral leaflet closure and opening, respectively, and the middle frame was used for measurements in mid-systole. In the apical 4-chamber view, the distance between the posterior papillary muscle and median portion of the mitral annulus was measured (posterior papillary muscle tethering length in the apical 4-chamber view; Figure 1 ). In the apical 2-chamber view, the distance between the anterior and posterior papillary muscles and the contralateral part of the mitral annulus were measured (anterior papillary muscle tethering length and posterior papillary muscle tethering length in the 2-chamber view, respectively; Figure 1 ).
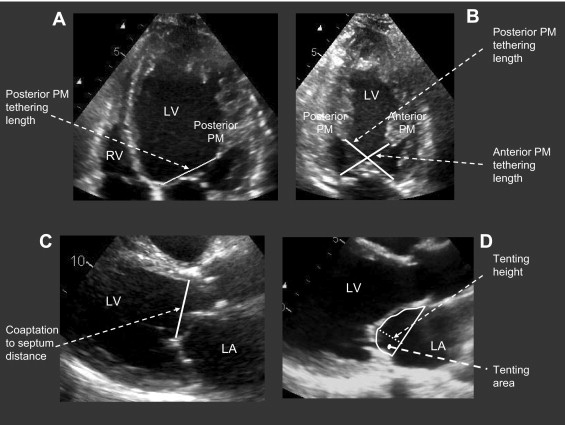
In the long-axis view, the coaptation-to-septum distance was measured in mid-systole, as the distance between the septum at the hinge point of the aortic valve cups and coaptation point of the mitral valve leaflets ( Figure 1 ). Evaluation of the geometry of the mitral valve was performed in mid-systole, as previously described. In the parasternal long-axis view, tenting area, tenting height, and coaptation length were measured. The tenting area was measured as the area enclosed between the annular line and the mitral leaflets, and the tenting height was defined as the distance between the coaptation point and the annular line ( Figure 1 ). The coaptation length was measured as the length of apposition of the anterior and posterior mitral leaflets.
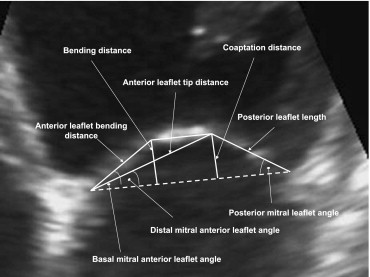
The tethering of mitral leaflets was estimated by calculation of the basal mitral anterior leaflet angle (ALA base ), the distal mitral anterior leaflet angle (ALA tip ), and the posterior mitral leaflet angle (PLA). The ALA base was defined as the angle between the annular plane and the basal portion of the anterior leaflet, and the PLA was defined as the angle between the annular plane and the posterior leaflet ( Figure 2 ). The ALA tip was defined as the angle between the annular plane and the anterior leaflet tip distance (which corresponded to the distance between the median part of mitral annulus and the coaptation point; Figure 2 ). The angles were calculated according to previously reported formulas. The mitral annular area was obtained from its dimensions in the 4- and 2-chamber views, using an ellipsoid assumption.
Operative risk was calculated according to EuroSCORE. Continuous data are presented as the mean ± SD and dichotomous data as the number of patients (percentage), as appropriate. Patients were divided into 2 groups according to the presence of MR at mid-term follow-up: patients without recurrent MR if no or mild MR was observed (effective orifice regurgitant area <0.20 cm 2 , regurgitant volume <30 ml/beat, and vena contracta <0.3 cm), and patients with recurrent MR if moderate or severe MR was observed (effective orifice regurgitant area ≥0.20 cm 2 , regurgitant volume ≥30 ml/beat, and vena contracta ≥0.3 cm). The preoperative and mid-term follow-up echocardiographic measurements of the patients without recurrent MR were compared to those of the patients with recurrent MR. The differences in clinical and echocardiographic baseline (preoperative) characteristics between the patients with and without recurrent MR were evaluated using Student’s t tests or the chi-square tests, as appropriate. Changes (from baseline to follow-up) in the echocardiographic data were analyzed by repeated measurements analysis of variance, and the interaction between the evaluation point (preoperatively vs during follow-up) and group (patients with recurrent MR vs patients without recurrent MR) was tested.
Univariate and multivariate Cox proportional hazard regression analyses were applied to further study the relation between the baseline characteristics and recurrent MR. On multivariate analysis, the stepwise backward method was used to identify the predictors of MR, with significant univariate predictors entered as covariates. The variables were checked for colinearity, and, if the correlation coefficient between 2 variables was >0.7, only 1 variable was retained in the model. A p value <0.05 was regarded as statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences for Windows, version 16.0 (SPSS, Chicago, Illinois).
Results
The demographic, clinical, and surgical characteristics of the patients are listed in Table 1 . The mean age was 62 ± 11 years, and 61% of patients were men. Most patients (85%) had New York Heart Association functional class III or IV. Patients were receiving optimal medical treatment for HF. No clinically relevant difference was found in the demographic or clinical characteristics between the patients with recurrent MR and those without recurrent MR at mid-term follow-up.
| Variable | All (n = 109) | MR at Follow-Up | |
|---|---|---|---|
| No (n = 88) | Yes (n = 21) | ||
| Age (years) | 62 ± 11 | 62 ± 11 | 62 ± 12 |
| Men | 66 (61%) | 54 (61%) | 12 (57%) |
| Body surface area (m 2 ) | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.3 |
| Systolic blood pressure (mm Hg) | 118 ± 24 | 118 ± 25 | 115 ± 19 |
| Diastolic blood pressure (mm Hg) | 72 ± 11 | 72 ± 12 | 70 ± 10 |
| New York Heart Association functional class | |||
| I | 2 (2%) | 1 (1%) | 1 (5%) |
| II | 14 (13%) | 11 (12%) | 3 (14%) |
| III | 77 (70%) | 63 (72%) | 14 (67%) |
| IV | 16 (15%) | 13 (15%) | 3 (14%) |
| Creatinine (μmol/L) | 110 ± 41 | 108 ± 36 | 116 ± 60 |
| Glomerular filtration rate (ml/min) | 61 ± 2 | 62 ± 22 | 58 ± 23 |
| Ischemic cardiomyopathy | 53 (49%) | 47 (53%) | 9 (43%) |
| Hypertension | 20 (18%) | 17 (20%) | 3 (14%) |
| Diabetes mellitus | 28 (26%) | 26 (30%) | 2 (10%) |
| Chronic obstructive pulmonary disease | 24 (22%) | 20 (23%) | 4 (19%) |
| Stroke | 6 (6%) | 6 (7%) | 0% |
| Peripheral vascular disease | 8 (7%) | 5 (6%) | 3 (14%) |
| LogEUROSCORE | 13 ± 11 | 14 ± 12 | 10 ± 7 |
| Medications | |||
| β Blockers | 81 (74%) | 66 (75%) | 15 (71%) |
| Calcium antagonists | 6 (6%) | 6 (7%) | 0% |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 89 (82%) | 73 (83%) | 16 (76%) |
| Diuretics | 92 (84%) | 74 (84%) | 18 (86%) |
| Spironolactone | 41 (38%) | 33 (38%) | 8 (38%) |
| Surgical characteristics | |||
| CorCap cardiac support device | 40 (37%) | 36 (41%) | 4 (19%) |
| Tricuspid valve plasty | 58 (53%) | 50 (57%) | 8 (38%) |
| Mitral valve ring (mm) | 26 ± 2 | 26 ± 2 | 26 ± 2 |
| Cardiopulmonary bypass (min) | 155 ± 45 | 151 ± 42 | 166 ± 54 |
| Aortic cross clamp time (min) | 93 ± 39 | 93 ± 38 | 95 ± 41 |
In 37% of patients, the CorCap cardiac support device was implanted concomitantly with restrictive mitral annuloplasty ( Table 1 ). The average mitral ring size used for annuloplasty was 26; in 53% of patients, tricuspid annuloplasty was also performed. Twelve patients had chronic atrial fibrillation and underwent a perioperative atrial fibrillation ablation procedure. No clinically relevant differences were found in the surgical characteristics between the patients with and without recurrent MR.
Preoperative echocardiographic quantitative analysis of MR revealed moderate to severe MR with a mean effective regurgitant orifice area of 0.33 cm 2 , regurgitant volume of 47 ml, and vena contracta of 5.5 mm ( Table 2 ).
| Variable | All (n = 109) | MR at Follow-Up | |
|---|---|---|---|
| No (n = 88) | Yes (n = 21) | ||
| Mitral regurgitation grade | |||
| 2 | 17 (15%) | 14 (16%) | 3 (14%) |
| 3 | 67 (62%) | 57 (65%) | 10 (48%) |
| 4 | 25 (23%) | 17 (19%) | 8 (38%) |
| Mean mitral regurgitation grade | 3.1 ± 0.6 | 3.0 ± 0.6 | 3.2 ± 0.7 |
| Effective regurgitant orifice (cm 2 ) | 0.33 ± 0.09 | 0.32 ± 0.09 | 0.35 ± 0.09 |
| Regurgitant volume (ml) | 47 ± 13 | 46 ± 13 | 50 ± 12 |
| Vena contracta (mm) | 5.5 ± 1.5 | 5.4 ± 1.2 | 6.0 ± 2.0 |
Of 122 patients, 10 died during the perioperative period, 112 were discharged from the hospital (with MR grade 0 to 1) and 3 patients died before the mid-term echocardiographic follow-up examination. In the final analysis, 109 patients with HF were included (56 with dilated cardiomyopathy and 53 with ischemic cardiomyopathy). All 109 patients underwent echocardiographic and clinical follow-up. At the mid-term follow-up, 88 patients (81%) continued to have MR grade 0 or 1, and MR greater than grade 2 was observed in 21 patients (19%). Of the patients with recurrent MR, grade 2, 3, and 4 MR was present in 14 (13%), 6 (5%) and 1 (1%), respectively.
The LV end-diastolic and end-systolic volume decreased from 217 ± 72 ml to 167 ± 61 ml and from 162 ± 65 ml to 119 ± 55 ml, respectively (p <0.001). The LV end-diastolic diameter decreased from 66 ± 8 mm to 60 ± 10 mm (p <0.001), and the LV end-systolic diameter decreased from 59 ± 9 mm to 52 ± 12 mm (p <0.001). A slight increase in the LV ejection fraction was observed (from 27 ± 9% to 31 ± 12%, p <0.001).
The preoperative MR severity was similar in patients with and without recurrent MR ( Table 2 ). In addition, no difference was noted in the preoperative LV and LA diameters or volumes ( Table 3 ). Concerning mitral valve geometry, patients with recurrent MR presented with greater preoperative ALA base , ALA tip , and PLA than the patients without recurrent MR (p <0.05; Table 4 ). In addition, patients with recurrent MR had a significantly increased tenting area and tenting height before surgery, and the coaptation length was lower than that in to the patients without recurrent MR (p <0.05; Table 4 ). Furthermore, the systolic and diastolic LV sphericity indexes were greater in patients with recurrent MR than those without recurrent MR (p <0.05; Table 4 ). The posterior and anterior papillary muscle tethering length and the coaptation-to-septum distance were similar in the patients with and without recurrent MR. Finally, the mitral annulus area was similar in both groups of patients.
| Variable | MR at Follow-Up | p Value (Time vs Group Comparison) | |||
|---|---|---|---|---|---|
| No (n = 88) | Yes (n = 21) | ||||
| Preoperative | Follow-Up | Preoperative | Follow-Up | ||
| Left atrial diameter (mm) | 45 ± 7 | 44 ± 7 ⁎ | 44 ± 6 | 45 ± 7 | NS |
| Left atrial volume (ml) | 93 ± 43 | 78 ± 38 ⁎ | 83 ± 27 | 87 ± 43 | 0.02 |
| Left ventricular ejection fraction (%) | 27 ± 9 | 32 ± 12 ⁎ | 28 ± 9 | 26 ± 8 | <0.01 |
| Left ventricular end-diastolic diameter (mm) | 66 ± 8 | 58 ± 10 ⁎ | 67 ± 10 | 66 ± 9 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 59 ± 8 | 51 ± 12 ⁎ | 60 ± 11 | 57 ± 11 | <0.01 |
| Left ventricular end-diastolic volume (ml) | 216 ± 65 | 159 ± 58 ⁎ | 218 ± 92 | 201 ± 66 | <0.001 |
| Left ventricular end-systolic volume (ml) | 161 ± 62 | 111 ± 51 ⁎ | 164 ± 82 | 151 ± 59 | <0.001 |
⁎ Corresponded to p <0.05 versus preoperative measurement in patients without recurrent MR; no difference noted in preoperative variables between patients with and without recurrent MR (p values not shown).
| Variable | MR at Follow-Up | p Value (Time vs Group Comparison) | |||
|---|---|---|---|---|---|
| No (n = 88) | Yes (n = 21) | ||||
| Preoperative | Follow-Up | Preoperative | Follow-Up | ||
| Mitral valve geometry | |||||
| Basal mitral anterior leaflet angle (°) | 31 ± 6 | 24 ± 6 ⁎ | 38 ± 5 ⁎ | 38 ± 5 | <0.001 |
| Distal mitral anterior leaflet angle (°) | 12 ± 3 | 11 ± 3 ⁎ | 19 ± 3 ⁎ | 19 ± 4 | <0.05 |
| Posterior mitral leaflet angle (°) | 40 ± 10 | 60 ± 11 ⁎ | 54 ± 9 ⁎ | 66 ± 9 † | 0.01 |
| Tenting area (cm 2 ) | 2.1 ± 0.3 | 1.4 ± 0.4 ⁎ | 2.9 ± 0.7 ⁎ | 2.1 ± 0.5 † | NS |
| Tenting height (mm) | 10.2 ± 1.2 | 9.1 ± 1.4 ⁎ | 12.6 ± 2.4 ⁎ | 12.0 ± 1.9 | NS |
| Coaptation length (mm) | 5.5 ± 1.2 | 8.7 ± 1.2 ⁎ | 4.9 ± 1.1 ⁎ | 6.7 ± 1.5 † | 0.001 |
| Mitral annulus (cm 2 ) | 10.1 ± 2.8 | 5.3 ± 1.5 ⁎ | 10.8 ± 2.9 | 7.0 ± 2.5 † | <0.001 |
| Left ventricular geometry | |||||
| Sphericity index diastole | 68 ± 9 | 60 ± 8* | 75 ± 8* | 71 ± 7 † | NS |
| Sphericity index systole | 65 ± 9 | 57 ± 9* | 72 ± 9* | 69 ± 8 | NS |
| Posterior papillary muscle length 4-chamber view (mm) | 41 ± 5 | 36 ± 5* | 43 ± 5 | 40 ± 4 † | NS |
| Posterior papillary muscle length 2-chamber view (mm) | 40 ± 6 | 33 ± 5* | 42 ± 5 | 37 ± 6 † | NS |
| Anterior papillary muscle length 2-chamber view (mm) | 40 ± 5 | 34 ± 5* | 42 ± 5 | 38 ± 5 † | <0.05 |
| Coaptation-to-septum distance (mm) | 40 ± 5 | 35 ± 5* | 42 ± 6 | 40 ± 5 † | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


