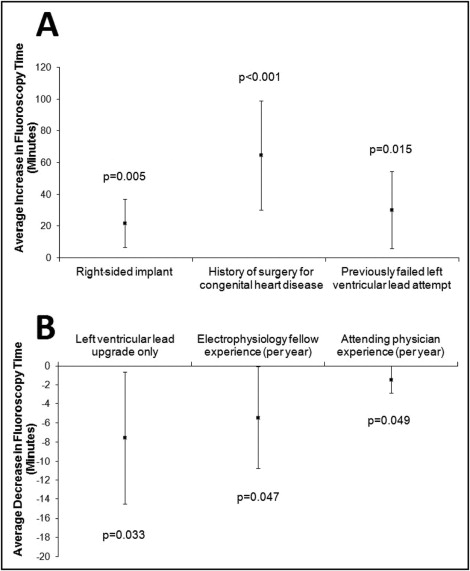
Biventricular device implantation with the insertion of a transvenous left ventricular (LV) lead can be challenging. The aim of this study was to identify predictors of procedural difficulty measured by fluoroscopy time and predictors of LV lead implantation failure. A single-center, retrospective study of 272 consecutive patients who underwent biventricular device implantation from 2004 to 2011 was conducted. Multivariate linear regression was used to assess predictors of fluoroscopy time and logistic regression to identify predictors of LV lead implant failure. The median fluoroscopy time was 36.1 minutes (interquartile range 24.2 to 51.6). After multivariate adjustment, independent predictors of longer fluoroscopy time included a right-sided approach (21.8 minutes longer, 95% confidence interval [CI] 6.8 to 36.9, p = 0.005), previous congenital heart disease surgery (64.6 minutes longer, 95% CI 30.2 to 99.0, p <0.001), and previous failed attempt (30.3 minutes longer, 95% CI 6.0 to 54.5, p = 0.015). Predictors of shorter fluoroscopy time included an LV lead upgrade (7.5 minutes shorter, 95% CI 0.6 to 14.4, p = 0.033), electrophysiology fellow experience (5.4 minutes shorter/year, 95% CI 0.1 to 10.7, p = 0.047), and attending physician experience (1.4 minutes shorter/year, 95% CI 0.01 to 2.9, p = 0.049). Failed implantation occurred in 8% of patients (22 of 272); inability to cannulate the coronary sinus and absent or atretic coronary sinus veins were the most common reasons (8 of 22 failed implants each). A previous failed attempt was the only significant predictor of LV lead implantation failure (odds ratio 33.5, 95% CI 3.2 to 352.6, p = 0.003). In conclusion, 6 patient and operator characteristics predicted LV lead implantation difficulty measured by fluoroscopy time. LV lead implantation failed in 8% of cases, predicted only by a previous failed attempt.
Congestive heart failure is responsible for significant morbidity and mortality. Biventricular (BiV) pacing with and without defibrillator therapy improves heart failure morbidity, quality of life, and survival in those with reduced left ventricular (LV) ejection fractions, heart failure symptoms, and increased QRS duration. However, BiV pacing requires the placement of an LV lead, which is associated with increased procedural difficulty and a higher rate of complications. LV lead placement failure occurs in 6% to 12% of implants, despite continuous improvements to LV lead implantation techniques and tools.
Even in cases with successful LV lead implantation, procedural difficulty during attempted implantation leads to longer procedure times, which increase the risk for device infection and expose the patient and operator to higher doses of radiation. With the premise that certain patient and operator characteristics may predict LV lead placement difficulty, our aim was to identify specific predictors of fluoroscopy time, a clinically relevant outcome measure. We also sought to identify predictors of LV lead implantation failure.
Methods
This was a single-center, retrospective cohort study of 272 consecutive patients who underwent LV lead implantation from 2004 to 2011. Patients who underwent de novo placement of a BiV device or upgrade to a BiV pacemaker or defibrillator were included.
Patients’ medical and surgical histories were obtained from chart review. Demographic data, including self-identified gender and race, were obtained from the electronic medical records. Lead, device, and intraprocedural data, including fluoroscopy time, procedure time, equipment used during LV lead placement, coronary sinus (CS) venography, and complications, were extracted from nursing records and the reports generated for the procedures. The study was approved by the Committee on Human Research of the University of California, San Francisco.
All BiV device and LV lead implantations were performed in dedicated electrophysiology laboratories by an electrophysiology fellow and attending physician, with the attending physician scrubbed during the procedure. After a subcutaneous pocket was created or reaccessed with blunt dissection, the axillary vein was accessed separately for each planned lead using a micropuncture introducer needle (Merit Medical, South Jordan, Utah) attached to a 10-ml syringe. For de novo BiV lead and device implantations, the right ventricular lead was implanted first. For LV lead implantation, CS ostium cannulation was attempted through a splittable safety sheath using a CS outer guide sheath. Choice of CS outer guide sheath, as well as whether a guidewire, inner sheath, and/or CS electrode catheter were used to aid cannulation, was at the operator’s discretion. Once CS access was obtained, CS venography was performed at the discretion of the operators: a cine loop venogram was saved by fluoroscopy in movie format (Witt Biomedical Corporation, Melbourne, Florida) during infusion of 10 ml of iodixanol (Visipaque; GE Healthcare, Princeton, New Jersey) through a balloon-tipped catheter. The LV lead was then advanced to the selected CS branch with or without the use of a thin 0.014-inch guidewire. Once satisfactory pacing and capture thresholds were deemed acceptable by the operator, the LV lead was tested for diaphragmatic stimulation with 10-V output. The CS sheath was then split and removed. In the absence of permanent atrial fibrillation or a preexisting right atrial lead, a right atrial lead was subsequently implanted. The leads were sutured to the muscle layer, screwed into the device generator, and as a unit were placed in the pocket, which was closed by suturing.
The primary outcome was the total fluoroscopy time used during BiV device and lead implantation. Total fluoroscopy time represented the cumulative time, in minutes, that radiography was turned on by the operator using a dead-man foot pedal switch. Total procedure time was also recorded and was measured from the instillation of local anesthetic to pocket closure. To assess more specifically the types of procedural difficulty encountered during LV lead placement, the total time devoted to LV lead placement was divided into 2 distinct intervals (secondary outcomes), called CS cannulation time (in minutes, from placement of the axillary vein sheath to stable engagement of the CS ostium with an outer guide sheath) and LV lead implantation time (in minutes, from stable engagement of the CS ostium with an outer guide sheath to anchoring of the LV lead with suture).
Continuous variables that were normally distributed (e.g., age, body mass index) are expressed as mean ± SD, whereas continuous variables not normally distributed are expressed as medians and interquartile ranges (IQRs). Categorical variables are expressed as percentages. Bivariate relations between dichotomous predictors and continuous outcomes of interest were examined using linear regression analysis. For predictor variables with >2 categories, analysis of variance was used. Bivariate relations between predictors and the dichotomous outcome of interest (LV lead implantation failure) were examined with logistic regression analysis. Multivariate linear regression analysis was performed to assess the independent predictors of the primary outcome (total fluoroscopy time), whereas multivariate logistic regression analysis was performed to assess the independent predictors of LV lead implantation failure. To determine which covariates to include in the stepwise algorithm for the final multivariate models, a group of likely predictors and confounders were specified a priori and included for face validity. Other possible predictors and confounders were generated using a directed acyclic graph, which was constructed from general clinical knowledge and data from previous studies. A backward-selection procedure was performed for each multivariate regression analysis, with a significance level for inclusion in the final model of <0.10. Only those covariates that remained independently associated with the outcome after adjustment in the final model were considered independent predictors. Two-tailed p values <0.05 were considered statistically significant. Stata version 11 (StataCorp LP, College Station, Texas) was used for statistical analysis.
Results
A total of 272 patients were included in the analysis. Baseline patient and operator characteristics are listed in Table 1 . Most patients were male and white. Most BiV placement attempts were performed on the patient’s left side and in patients without previous device therapy. The median total fluoroscopy time for the cohort was 36.1 minutes (IQR 24.2 to 51.6), while the median total procedure time was 199.9 minutes (IQR 159.2 to 239.0).
| Characteristic | Value |
|---|---|
| Age (years) | 64 ± 14.6 |
| Male | 198 (72.8%) |
| Race | |
| White | 173 (63.8%) |
| Black | 20 (7.4%) |
| Asian | 28 (10.3%) |
| Native American | 1 (0.4%) |
| Other | 49 (18.1%) |
| Body mass index (kg/m 2 ) | 27.8 ± 6.6 |
| Ischemic cardiomyopathy | 118 (43.4%) |
| Previous myocardial infarction | 47 (17.3%) |
| Hypertension | 88 (32.5%) |
| Diabetes | 54 (19.9%) |
| Atrial fibrillation | 86 (31.6%) |
| Coronary artery bypass surgery | 60 (22.1%) |
| Mitral valve replacement | 9 (3.3%) |
| Mitral valve repair | 4 (1.5%) |
| Congenital heart disease surgery | 3 (1.1%) |
| Previous other cardiothoracic surgery | 18 (6.6%) |
| Electrocardiographic QRS duration (ms) | 148 ± 33.8 |
| LV ejection fraction (%) | 29.9 ± 11.7 |
| Attending physician experience (days) | 1,470. 3 ± 902.8 |
| Electrophysiology fellow experience (days) | 356.3 ± 220.7 |
| Failed previous LV lead attempt | 6 (2.2%) |
| Upgrade procedure | |
| Previous pacemaker | 35 (12.9%) |
| Previous implantable cardioverter-defibrillator | 58 (21.3%) |
| Right-sided implantation | 11 (4.0%) |
| Device and lead manufacturer | |
| St. Jude Medical | 119 (43.8%) |
| Medtronic | 80 (29.4%) |
| Boston Scientific | 68 (25.0%) |
| Biotronik | 5 (1.8%) |
Table 2 lists the unadjusted analysis of predictors of total fluoroscopy time during BiV device implantation. After adjustment for covariates in the final model, patients with a right-sided approach, a history of surgery for congenital heart disease, and a previous failed attempt required significantly longer fluoroscopy times during BiV device implantation ( Figure 1 ). An LV lead upgrade procedure, electrophysiology fellow experience, and attending physician experience were associated with shorter fluoroscopy time during LV lead implantation ( Figure 1 ). Calendar year of implantation, the LV ejection fraction, and device manufacturer were not associated with fluoroscopy time in bivariate analysis (p = 0.53, p = 0.90, and p = 0.12, respectively), and inclusion of calendar year of implantation, the LV ejection fraction, and device manufacturer in the backward stepwise selection algorithm did not change the final predictors of fluoroscopy time. Additionally, a sensitivity analysis restricted only to de novo BiV implantations (i.e., excluding the upgrade procedures) did not meaningfully change the overall results. In patients with additional leads implanted other than LV leads, there was no statistically significant difference in fluoroscopy time for those implanted with right ventricular and right atrial leads compared to those implanted with right ventricular leads only (p = 0.28).
| Variable | β ⁎ (95% CI) | p Value |
|---|---|---|
| Right-sided implantation | 19.6 (4.2 to 35.0) | 0.013 |
| History of surgery for congenital heart disease | 60.6 (32.2 to 89.1) | <0.001 |
| Previous failed LV lead attempt | 10.3 (−10.6 to 31.2) | 0.33 |
| LV lead upgrade procedure only | −6.8 (−13.4 to −0.3) | 0.041 |
| Electrophysiology fellow experience (per year) | −4.6 (−9.8 to 0.06) | 0.08 |
| Attending physician experience (per year) | −0.9 (−2.2 to 0.03) | 0.15 |
⁎ The β coefficient represents the change in minutes of fluoroscopy time (positive values denoting more minutes of fluoroscopy time and negative values denoting fewer minutes of fluoroscopy time) per 1-unit increases of continuous predictor variables or as associated with individual categorical variables.
LV lead implantation failure occurred in 22 of 272 patients (8.1%) because of difficulty in cannulating the CS ostium (8 of 22 [36.4%]), absent or atretic CS vein anatomy precluding lead placement (8 of 22 [36.4%]), inability to achieve adequate pacing threshold (4 of 22 [18.2%]), and lead instability (2 of 22 [9.1%]). In 3 of 22 failed LV lead implantations, previous attempts had failed.
The only significant predictor of LV lead implantation failure in the final multivariate model was a previous failed attempt (adjusted odds ratio 33.5, 95% confidence interval [CI] 3.2 to 352.6, p = 0.003). Although a higher body mass index was associated with LV lead implantation failure in bivariate analysis, this was no longer significant after multivariable adjustment (adjusted odds ratio 1.1, 95% CI 1.0 to 1.1, p = 0.55).
The median CS cannulation time was 10.1 minutes (IQR 6.3 to 16.1) in the 203 patients with available data. In bivariate analysis, CS cannulation time was closely associated with fluoroscopy time; each 1-minute increase in CS cannulation time was associated with a 0.6-minute increase in fluoroscopy time (95% CI 0.4 to 0.7 minutes, p <0.001). In a multivariate model including the 6 predictors independently associated with the primary outcome of fluoroscopy time, only the side of implantation affected CS cannulation time: right-sided implantation was associated with a longer CS cannulation time (26.6 minutes longer, 95% CI 9.5 to 43.8, p = 0.003).
A CS electrode catheter was used to aid CS cannulation in 177 patients (69.4%). In most patients (n = 219 [85.9%]), 1 outer guide sheath was used. For the rest of the population, a total of 2 (n = 21 [8.2%]), 3 (n = 8 [3.1%]), or 4 (n = 7 [2.8%]) outer sheaths were used. In bivariate analysis, each additional outer sheath used was associated with an increased CS cannulation time (25.5 minutes longer, 95% CI 22.3 to 28.8, p <0.001) and fluoroscopy time (15.0 minutes longer, 95% CI 10.5 to 19.5, p <0.001). In a multivariate model including the 6 predictors associated with fluoroscopy time, only right-sided implantation was associated with the use of additional outer sheaths (0.6 sheaths, 95% CI 0.2 to 1.0, p = 0.006).
After CS cannulation, the median LV lead implantation time was 37.8 minutes (IQR 23.3 to 62.8) in the 201 patients with available data. In bivariate analysis, LV lead implantation time was also associated with fluoroscopy time; each 1-minute increase in LV lead implantation time was associated with a 0.4-minute increase in fluoroscopy time (95% CI 0.3 to 0.5 minutes, p <0.001). In a multivariate model including the 6 predictors associated with the primary outcome of fluoroscopy time, only a history of congenital heart disease surgery was associated with a longer LV lead implantation time (149.1 minutes longer, 95% CI 93.7 to 204.5, p <0.001).
CS venography was performed in 240 patients (88.6%). Use of CS venography was not associated with a difference in fluoroscopy time (p = 0.17). During LV lead implantation attempts, 1 or no 0.014-inch wires were used in 162 patients (63.5%). In the rest of the cohort, 2 or 3 (n = 77 [30.2%]), 4 or 5 (n = 13 [5.1%]) or ≥6 (n = 3 [1.2%]) wires were used. In bivariate analysis, each additional wire used was associated with an increased LV lead implantation time (12.6 minutes longer, 95% CI 8.1 to 17.0, p <0.01) and an increased fluoroscopy time (7.0 minutes longer, 95% CI 4.6 to 9.4, p <0.001). In a multivariate model including the 6 predictors associated with fluoroscopy time, again, only a history of congenital heart disease surgery was associated with use of additional wires (2.4 wires, 95% CI 0.6 to 4.2, p = 0.008).
Traumatic CS complications occurred in 10 patients, including CS perforation (n = 3 [1.1%]), CS dissection (n = 6 [2.2%]), and both CS perforation and dissection (n = 1 [0.4%]). None of these patients required pericardiocentesis or urgent cardiac surgery, and there were no deaths from traumatic CS complications. Failure of LV lead placement occurred in only 1 of these patients, because of difficulty in stably cannulating the CS ostium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


